Dr Bettina Ryll from the Melanoma Patient Network Europe gives a patient advocacy perspective
The market approval of Ipilimumab in 2011 marked the begin of a new era for advanced Melanoma and the following five years and 10 further therapies revolutionised the treatment and the outlook of a cancer previously only known for its dire prognosis.
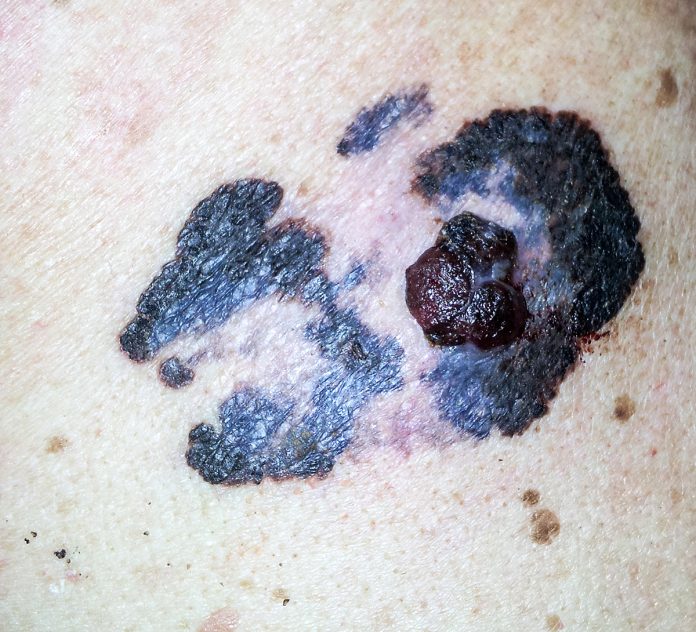
Melanoma is a cancer starting from the body’s pigment-producing cells. Often labelled as ‘skin cancer’, this is the place where it most frequently starts. In rarer instances, however, melanoma also occurs in the eye, the inner linings of the body and the wrappings of the brain. Once melanoma spreads, a patient can only expect to survive six to nine months without one or several of the new therapies. Detected early, melanoma is usually considered curable by surgery. However, recent data demonstrates that even the smallest melanomas can recur over a long period of time and over 20 years will cost 15-30% patients their lives. With incidences rising worldwide, effective prevention, early detection and accessible treatments will, therefore, remain critical to reduce the mortality of this disease.
In the past, melanoma patient advocacy in Europe was fragmented and isolated. In the absence of effective therapies, the focus was on prevention, early detection and emotional support. The arrival of promising new therapies also triggered an evolution of European melanoma patient advocacy as patients suddenly had hope but struggled to evaluate and access new therapies.
The best cancer is the one you don’t get – when effective prevention clashes with beauty ideals
Excessive exposure to UV-light and a history of sunburn in childhood are major risk factors for developing melanoma later in life. Sun-safe behaviour, in particular for children, is, therefore, one of the most effective preventive measures. Unfortunately, and despite increasing media coverage about the risks of melanoma, ‘having a tan’ remains associated with holidays abroad, leisure time and beauty. If anything, this demonstrates once more that knowledge and information alone are insufficient to induce behavioural change but need to be underpinned with legal frameworks and policies, for example, regarding sunbed use, the provision of shaded play areas for children and the ban of advertisements promoting sun-risky behaviour.
If you cannot prevent it, find it as early as possible – a call for effective early detection
Not every melanoma is preventable and past damage cannot be undone. Detecting a melanoma a single millimetre smaller can considerably impact a patient’s chances of survival. However, screening programmes come with their own challenges. The availability and quality of skin examination programs vary greatly between and even within countries, from whole-body inspection with mole-mapping and dermoscopy, to the ad-hoc visual inspection of lesions pointed out by the patient.
Education in self-examination, strategies how to reach groups at particular risk, the broad availability of high-quality skin screening programmes and the recognition that the proactive removal of suspect moles helps to reduce human suffering as well as cost in the long-term, will help to reduce the impact of what remains a deadly disease.
A therapeutic revolution in melanoma – albeit at considerable human cost
Prior to the arrival of the latest therapies, only 15% of patients with metastatic melanoma could expect to be alive after two years – compared to over 60% after three years on certain therapies today. While this development was met with incredulous awe and filled plenary sessions at major congresses, this revolution also had its casualties.
Unfortunately, scientific progress was not met by progress of regulatory and health-economic methodologies of the same speed as the new melanoma therapies belonging to new therapeutic classes did not behave like traditional oncology products. Surprising efficacy in a situation of high unmet need, combined with unexpected patterns and timing of responses and toxicity clearly demonstrated the limitations of traditional RCTs (randomised controlled trials), a methodology originally developed to detect small to medium differences in large unselected patient populations treated with rather unspecific therapies.
Large effect sizes over the ‘standard of care’ DTIC known for its limited effectiveness, meant that the only option for melanoma patients to access new therapies was in equipoise-violating trials, challenging the concept of free choice in the face of certain death.
Large effect sizes also meant that the resulting RCTs were small with very restrictive entry criteria.For example, patients with brain metastases were initially systematically excluded – and that despite the fact that metastasis to the brain is the main driver of mortality in melanoma. This first limited access to clinical trials for desperate patients, and then later restrictive reimbursement due to the limited external validity of the results.
As pharmacovigilance (safety of medicines) operates according to different statistical principles, small clinical trials also meant limited safety information and rarer side effects continue to present a considerable challenge in the management of melanoma.
The challenge of dealing with these novel therapies found its reflection in inappropriate trial designs, delays in approval and reimbursement – all potentiated by their high cost. Ultimately, this led to the loss of patients lives that could have been saved and seriously questions our ability to successfully drive and adapt innovation for the benefit of European citizens.
The hope of surviving an otherwise deadly disease – together with the learnings from the HIV community that had successfully challenged existing drug development paradigms in the past- and greatly facilitated by today’s internet tools for education, communication and collaboration initiated a rapidly growing European network of melanoma patients and carers that were no longer willing to simply accept the status quo, and is now known as MPNE, the Melanoma Patient Network Europe.
Today, MPNE is a multi-dimensional network system based on language, national or topic-specific interests and centred around an English-speaking core. With a substantial focus on education, MPNE allows melanoma patients to follow and act upon the latest developments in the disease and routinely covers major scientific events, like ASCO and ESMO. Motivated by the personal experience of the limitations of current drug development, MPNE is involved in initiatives on patient-centric clinical trial designs, innovation in regulation and health technology assessment and sustainable healthcare innovation in the hope that patients with other equally desperate disease benefit from and do not have to repeat the learnings in melanoma.
Please note: this is a commercial profile
Dr Bettina Ryll
Melanoma Patient Network Europe