Dr Carlos Ziebert, head of IAM-AWP’s Calorimeter Centre, KIT, outlines how research and testing in calorimeters paves the way for safer batteries
Established in 2011, the Calorimeter Centre at the Karlsruhe Institute of Technology’s (KIT) Institute for Applied Materials – Applied Materials Physics, operates Europe’s largest battery calorimeter laboratory. It provides six Accelerating Rate Calorimeters (ARCs) of different sizes – from coin to large pouch or prismatic automotive format – which allow the evaluation of thermodynamic, thermal and safety data for lithium-ion cells on material, cell and pack level under quasiadiabatic and isoperibolic environments for both normal and abuse conditions (thermal, electrical, mechanical).
Safety of Li-ion batteries
Safety comes first – This is the mission of the centre’s head, Dr Carlos Ziebert. A holistic safety assessment is a prerequisite for upscaling and market acceptance of battery technologies, because the thermal runaway can cause ignition or even explosion of the cell that leads to negative public attention or even rejection. With increasing energy density, the safety of Li-ion batteries is becoming more and more important. Thus, calorimetry is a fundamental technique in order to obtain quantitative data on the thermal and safety behaviour – you need to know how many watts a cell will produce under every condition. This information can then be used to adapt the battery management, thermal management and safety systems. In the ARCs the temperature, heat and internal pressure evolution can be studied, while operating cells under conditions of normal use, abuse or accidents. The battery calorimeters provide thermal stability data on materials level, eg of anodes, cathodes or electrolytes or their combinations and safety tests on cell and pack level by applying.
- a) Electrical abuse: External/internal short circuit test, overcharge test, overdischarge test
In the ARC, the temperature increase by applying an external short circuit or during an internal short circuit, which might be caused eg by a production fault, can be measured. The cell can also be overcharged or overdischarged, leading to different failure modes.
- b) Mechanical abuse: Nail test
In the ARCs, a mechanical system allows to push a nail into the cell. This provides not only a pass/fail type test to qualify cells, but also quantitative data.
- c) Thermal abuse: Heat-Wait-Seek test, ramp heating test, thermal propagation test
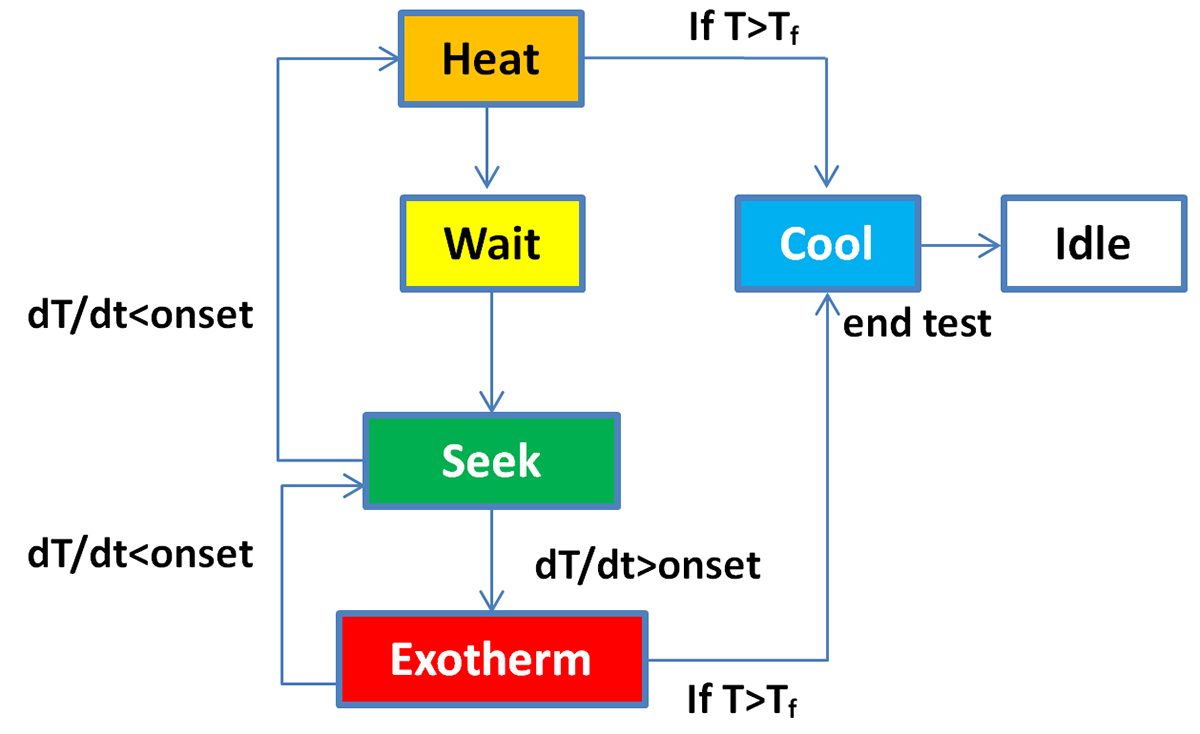
The Heat-Wait-Seek (HWS) test starts in the Heat Mode by heating up the cell in small temperature steps (typically 5K), as shown in the schematic flowchart in Fig.1.
At the end of each step, the Wait Mode is activated to reach thermal equilibrium. Then the system enters Seek Mode, which seeks the temperature rate and ends with two possible modes: Exotherm Mode or Heat Mode. If the measured temperature rate is larger than the onset sensitivity (typically 0.02K/min), which means that self-heating of the cell is detected, the system goes into Exotherm Mode.
In this quasiadiabatic mode, the heaters in the calorimeter chamber follow immediately any change of the cell temperature, preventing the heat transfer to the chamber. Thus, it is heating up more and more until a thermal runaway occurs or the chemicals are completely consumed by the exothermic reactions.
On the other hand, if the temperature rate is smaller, the system goes back into Heat Mode. If the temperature exceeds the end temperature value, the ARC enters the Cool Mode, switches off the heaters and starts to cool down by introducing pressurised air to the chamber. For the Ramp Heating test, which mimics a Hot Box test, the cells are heated up at a constant rate instead of a stepwise heating of the cells as in the HWS test.
Thermal runaway
In addition, new methods for the measurement of external and internal cell pressures for early prediction of thermal runaway of LIB have been established on 18650 cells.
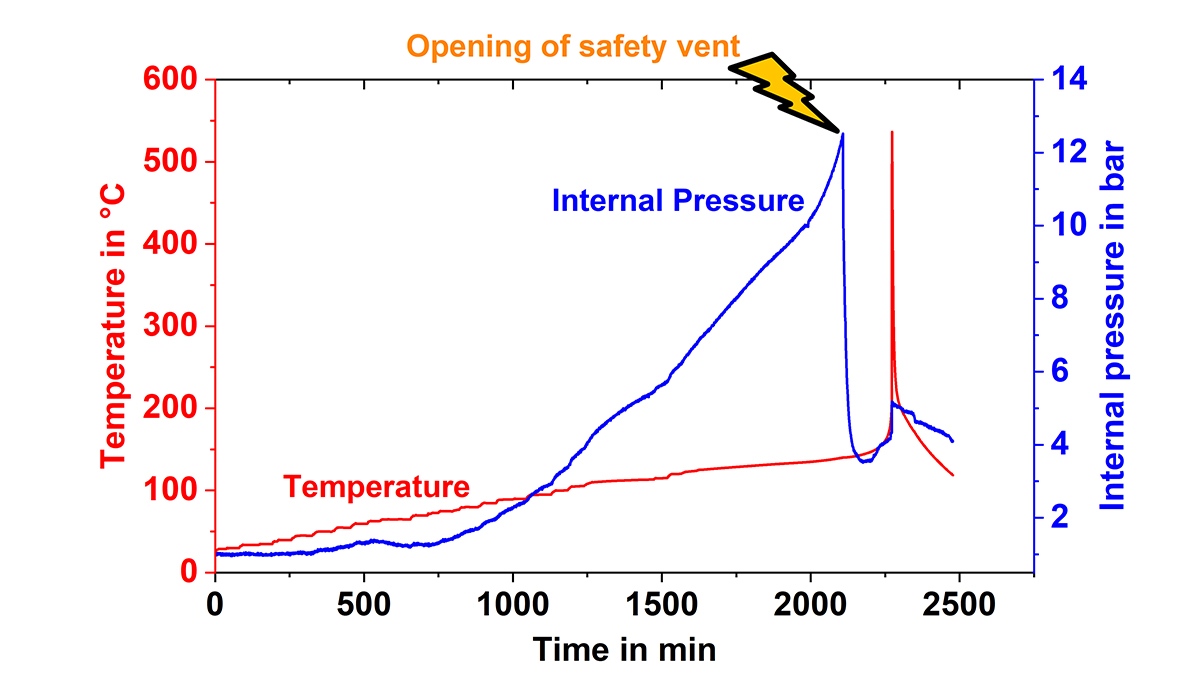
The external pressure was measured using a gas-tight cylinder inside the calorimeter chamber, in order to detect the venting of the cells. For internal pressure measurements, a pressure line connected to a pressure transducer was directly inserted into the cell and the pressure was recorded during a HWS test, as can be seen in Fig.2. This plot clearly shows that, at first, a pressure increase occurs much earlier than a self-heating and, at second, that the cell goes into thermal runaway even if the safety vent opens and releases gases leading to pressure drop. Thus, the measurement of the internal pressure could be used for the early prediction of processes leading to thermal runaway. This method has been adapted from cylindrical cells to pouch cells and prismatic automotive cells.
Moreover, the calorimeters allow studying the thermal runaway propagation in order to develop and qualify suitable countermeasures, such as heat protection barriers.
As result of the battery calorimeter tests quantitative and system relevant data for temperature, heat and pressure development of the cells as well of the decomposition products are provided as: i) a fast feedback for the cell developers, ii) essential data for modeling and simulation and iii) required data for the design and adaptation of battery management.
Thus we hope that the Calorimeter Center in close cooperation with the EERA (European Energy Research Alliance) Joint Programme on Energy Storage will help to establish a European cell production with a world-leading technology, as it is aimed with the European Battery Alliance and the Battery 2030+ initiative.
Please note: This is a commercial profile