Leading independent European antisense drug discovery company, Secarna, aims to bring novel and effective antisense therapeutic options to an increasing number of patients. CEO Alexander Gebauer tells us how
Secarna Pharmaceuticals is a next-generation antisense oligonucleotide (ASO) company that addresses disease areas of high unmet medical need, focusing on immuno-oncology and inflammation/fibrosis-related indications.
Founded in 2015, Secarna is proud to have established its discovery and development unit in over 2,000 square feet of lab and office space at the IZB Martinsried, Munich – one of Europe’s most vibrant biotechnology clusters. Alexander Gebauer, CEO of Secarna, explains why the company’s best-in-class ASO platform, LNAplus™, combined with its strategic business model, offers Secarna an advantageous position to establish ASOs as the third pillar, together with small molecules and antibodies, of drug development, expanding the universe of targetable genes.
Exploring Secarna’s business model
Secarna’s strategy builds on two pillars: our partnering approach based on our unique technology platform, where we jointly work on the discovery and development of antisense oligonucleotides in disease-focus areas for our respective partners, and our growing proprietary pipeline, which is focused on areas of high unmet medical need such as immuno-oncology and inflammation/fibrosis.
We plan to partner our proprietary projects after reaching certain value inflection points.
How does Secarna’s technology stand out from other antisense therapeutics? What are the company’s core expertise?
Over time, and with our extensive experience, we have built a highly versatile, effective, and valuable platform encompassing all aspects of drug discovery and preclinical development, delivering high-quality candidates quickly.
From the inception of each project, we focus on simultaneously assessing both efficacy and safety. Our proprietary Oligofyer bioinformatics system allows us to thoroughly screen all relevant databases and select the most promising antisense oligonucleotides in-silico, minimising off-target effects and potentially toxic sequences at a very early stage.
We set the bar very high for each project; therefore, we exclusively work on so-called third-generation chemistry ASOs, with improved stability, target affinity, pharmacokinetic properties and productive cellular uptake. Moreover, our molecules show no or reduced unwanted immune system stimulation and other possible side effects.
What do you believe are the advantages/disadvantages that ASOs have over other therapies? Which indications might profit from them the most?
Like other compound classes, such as small molecules or antibody therapies, antisense oligonucleotides are not a miracle solution for every therapeutic problem. However, we see clear advantages over other modalities for many targets.
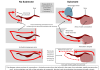
Therapeutic antibodies cannot address intracellular targets, small molecules cannot be designed for every target, and siRNAs require transport technologies, altogether highlighting the need for tailored approaches, depending on the indication. We believe that ASOs offer a particular advantage over other drug modalities; for example, in contrast to siRNAs, they can be applied ‘naked’ and still enable potent target knockdown in various organs and tissues.
Also, they achieve specific blockage of all functions of a selected target due to pre-messenger RNA (mRNA) targeting, whereas an antibody might block only one epitope.
Furthermore, they can target all types of proteins (e.g., intracellular, secreted, surface), making them relevant to treat a wide range of diseases, where the liver, kidney, central nervous system (CNS), lung or other organs are affected. ASOs are also acquiring a relevant role in oncology, bringing the immuno-oncology space to the focus of our proprietary pipeline.
How is Secarna’s pipeline structured?
While we can share only limited information about our partnered programmes, it is public that Denali and SciNeuro are both CNS companies. Lipigon is currently in phase 1 clinical trials with its cardiometabolic antisense oligonucleotide project. Immuno-oncology and inflammation/fibrosis are key areas of interest in our internal pipeline.
Is there an indication that Secarna has a stronger position?
Our main areas of scientific expertise are oncology, inflammation, and CNS, backed by a strong network of key opinion leaders that support us and advise us in the development of our current programmes and partnerships.
Nonetheless, we have the capability to quickly expand our therapeutic range if required.
What are the key factors that make a successful partnership? What type of partnerships is Secarna looking for?
Besides scientific and operational excellence, a successful partnership requires efficiently working with the partner’s scientific team and building a trustful collaboration model.
At Secarna, we pride ourselves on our versatile business model to match our partner’s needs, demonstrated by a track record of fruitful and robust partnerships.
What is your vision for the company for the next 5-10 years, and Secarna’s plans for the development of its internal programmes?
We plan to become the global partner of choice for all antisense oligonucleotide projects. Therefore, we continue to refine and update our discovery and development engine and invest in our knowledge to be at the forefront of understanding the underlying science behind ASOs.
Our vision is that in a few years, we will contribute significantly to expanding the therapeutic options against currently undertreated diseases. Our partnered programmes and in-house projects will hopefully offer more patients new and excellent therapeutic options. Either via external capital or partnering, several of our internal projects are expected to reach the IND-ready and clinical development stages by 2024/25.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.