Prof. Dr. rer. nat. Regina Fluhrer discusses Signal Peptide Peptidase-Like proteases and their role in cellular signalling and metabolism

Signal Peptide Peptidase-Like (SPPL) proteases are intramembrane proteases that have their catalytic centres embedded in cellular membranes. This allows them to cleave other membrane proteins, their substrates, in the special environment of cellular membranes (for more details see October 2018 edition). This cleavage results in the release of protein fragments to both sides of the membrane, which are able to translocate to other parts of the cell where they may act as messenger molecules and transfer signals in order to regulate certain cellular processes (for more details see January 2019 edition). Applying genomic searches SPPL proteases have been discovered more than 15 years ago as a homologue of Signal Peptide Peptidase (SPP), an intramembrane protease that is involved in maturation of other membrane proteins and in coping cellular stress. However, only recently have we started to understand the physiological functions of these fascinating proteases.
SPPL3 the smallest member of the SPPL proteases localizes to the membrane of the Golgi apparatus, a cellular compartment that is responsible for sorting and maturation of integral membrane proteins. A peculiarity of membrane proteins are sugar moieties that are attached to their extracellular domains on their way to the cell surface. They are not only important for stability and the three-dimensional configuration of these proteins, but also impact on their function. The sugar moieties of membrane proteins integrated in the plasma membrane, that delimits a cell from its surrounding, are important players of the so called extracellular matrix and key for cell to cell communication within organs. We recently found that SPPL3 is capable of cleaving glycosyltransferases, those enzymes that transfer the sugar moieties on the membrane proteins. This cleavage results in release and secretion of the glycosyltransferase domain which comprises the active centre crucial for transfer of the sugar molecules and, thus, reduces the activity of the glycosyltransferases in the Golgi (Figure 1). Consequently, protein glycosylation and thus, also the composition of the extracellular matrix can be regulated via the amount of SPPL3 present in the cell – the more SPPL3 is expressed, the less protein glycosylation occurs and vice versa. Changes in the glycan composition of membrane proteins affect physiological processes like signalling and cell growth, but also pathological processes like angiogenesis or tumor metastasis. Therefore, SPPL3 expression may be an attractive cellular switch to regulate such processes. Based on the fact that proteolytic cleavages are irreversible, it must be assumed that SPPL3 expression under physiological conditions is tightly regulated. Thus, to further pursue SPPL3 as promising drug target, a precise understanding of these regulatory mechanisms is indispensable and in the focus of current research activities.
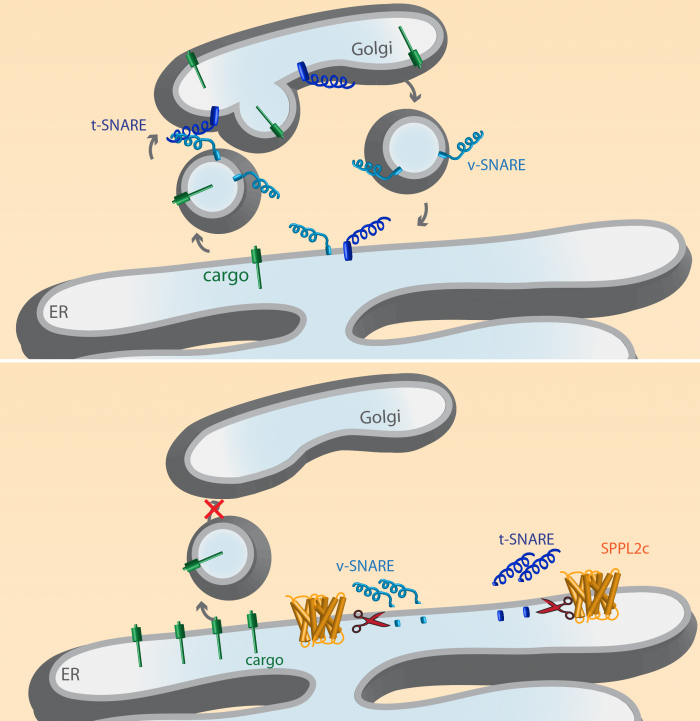
Another member of the SPPL protease family, the Signal-Peptide-Peptidase like 2c (SPPL2c) protease is selectively expressed in the testis, where it cleaves membrane anchored proteins. Among them phospholamban, a protein involved in Calcium-signalling and the so-called SNARE proteins that are crucial for cargo transport between the individual cellular compartments. SPPL2c cleavage of SNARE proteins blocks transport between compartments (Figure 2) and would be detrimental for most cells. In the maturating sperm it, however, helps to reorganize the cellular compartments which is crucial for proper sperm maturation.
These examples illustrate that SPPL proteases are involved in key cellular pathways and may have the potential to become targets for diseases for which to date no cure exists. However, to utilize SPPL proteases in the future as targets to treat or even cure diseases, it is indispensable to understand their cleavage mechanisms, substrate repertoires and physiological function in depth. Therefore, a lot more basic research on these fascinating enzymes will be required to raise this treasure.
Prof. Dr. rer. nat. Regina Fluhrer
Lehrstuhl für Biochemie und Molekularbiologie
Medizinische Fakultät Universität Augsburg
Universitätsstraße 2
86135 Augsburg
Tel: 0049 821 598 – 2069
regina.fluhrer@med.uni-augsburg.de
Further reading:
Fluhrer R. A Unique Family of Intramembrane Proteases. Scientia. 2018 Jul 04; 179: 10.26320/SCIENTIA179
Voss M, et al. Shedding of glycan-modifying enzymes by signal peptide peptidase-like 3 (SPPL3) regulates cellular N-glycosylation. EMBO J. 2014 Dec 17;33(24):2890-905.
Niemeyer J, et al. The intramembrane protease SPPL2c promotes male germ cell development by cleaving phospholamban. EMBO Rep. 2019 Mar;20(3).
Papadopoulou AA, et al. Signal Peptide Peptidase-Like 2c (SPPL2c) impairs vesicular transport and cleavage of SNARE proteins. EMBO Rep. 2019 Mar;20(3).