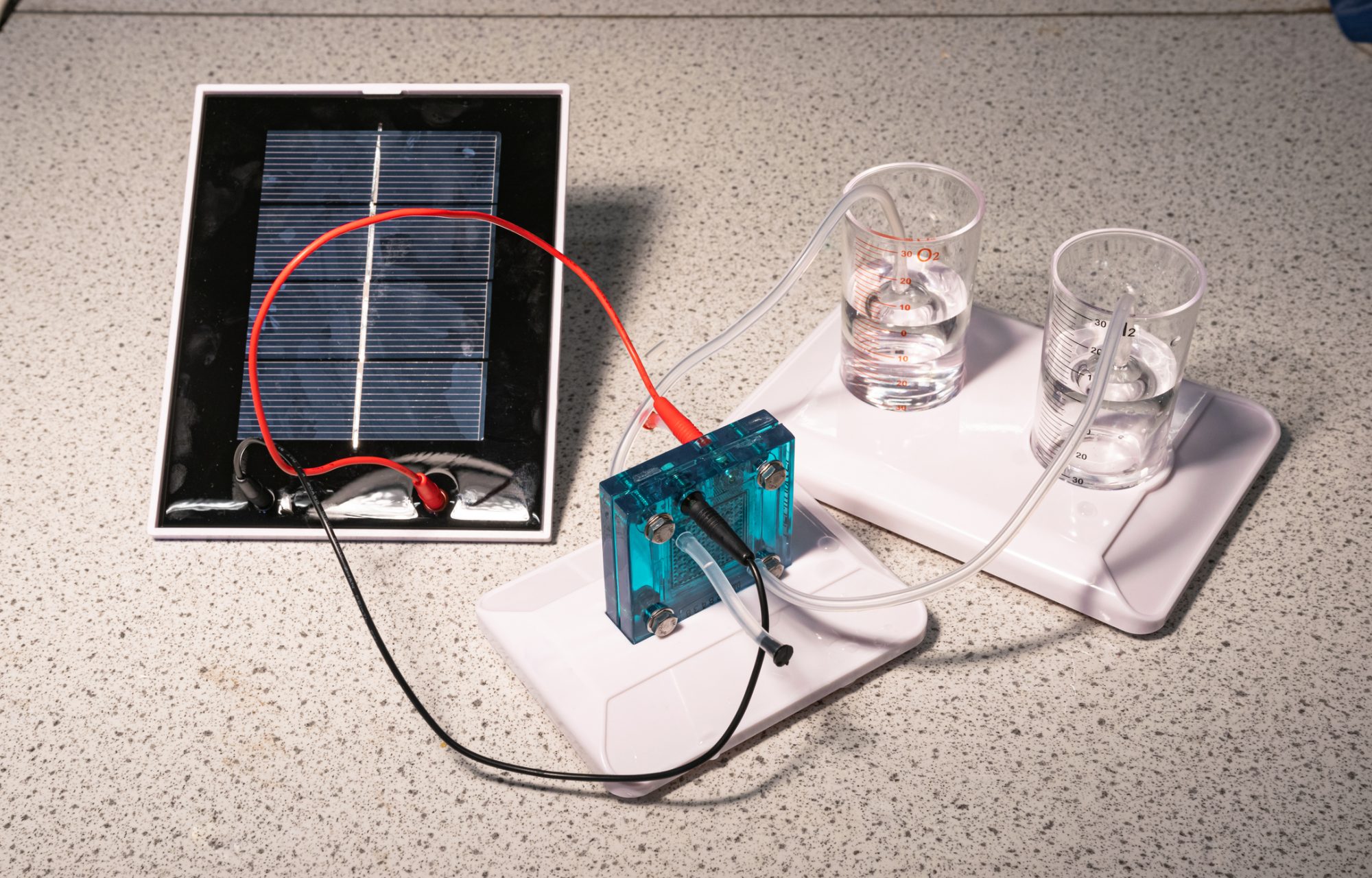
A new kind of solar panel has achieved 9% efficiency in converting water into solar powered hydrogen and oxygen, mimicking natural photosynthesis
Currently, humans produce hydrogen from the fossil fuel methane, which uses a lot of fossil energy in the process. However, plants can harvest hydrogen atoms from water using sunlight, and researchers have been looking into solar powered hydrogen.
In order to reduce global carbon emissions, hydrogen is a viable alternative and a component of sustainable fuels made with recycled carbon dioxide. It is needed for many chemical processes, such as fertiliser production.
This new solar powered hydrogen, developed at the University of Michigan, could drive down the cost of sustainable hydrogen – done by shrinking the semiconductor, which is typically the most expensive part of the device.
This semiconductor withstands concentrated light equivalent to 160 suns
Withstanding high temperatures and the light of 160 suns, a new catalyst is 10 times more efficient than previous sun-powered water-splitting devices of its kind.
This result was generated by two advances: the ability to concentrate the sunlight without destroying the semiconductor that harnesses the light, and using both the higher energy part of the solar spectrum to split water and the lower part of the spectrum to provide heat that encourages the reaction.
Finally, this is enabled by a semiconductor catalyst that improves itself with use, resisting the degradation that such catalysts usually experience when they harness sunlight to drive chemical reactions.
“Hydrogen produced by our technology could be very cheap”
Peng Zhou, U-M research fellow in electrical and computer engineering, said: “We reduced the size of the semiconductor by more than 100 times compared to some semiconductors only working at low light intensity. Hydrogen produced by our technology could be very cheap.”
Zetian Mi, U-M professor of electrical and computer engineering, added: “In the end, we believe that artificial photosynthesis devices will be much more efficient than natural photosynthesis, which will provide a path toward carbon neutrality.”
Nearly 10 times more efficient than other solar water-splitting experiments
This solar powered hydrogen developer can thrive in high temperatures that are punishing to computer semiconductors.
Higher temperatures speed up the water splitting process, and the extra heat also encourages the hydrogen and oxygen to remain separate rather than renewing their bonds and forming water once more – both of these can help to harvest more hydrogen.
For the outdoor experiment, the researchers set up a lens to focus sunlight onto an experimental panel just a few inches across. Within that panel, the semiconductor catalyst was covered in a layer of water, bubbling with the hydrogen and oxygen gasses it separated.
There was a 6.1% efficiency turning the energy from the sun into hydrogen fuel
The catalyst is made of indium gallium nitride nanostructures, grown onto a silicon surface. That semiconductor wafer captures the light, converting it into free electrons and holes – positively charged gaps left behind when electrons are liberated by the light.
The nanostructures are peppered with nanoscale balls of metal, 1/2000th of a millimeter across, that use those electrons and holes to help direct the reaction.
Higher temperatures speed up the water splitting process
A simple insulating layer atop the panel keeps the temperature at a toasty 75 degrees Celsius, or 167 degrees Fahrenheit, warm enough to help encourage the reaction while also being cool enough for the semiconductor catalyst to perform well.
The outdoor version of the experiment, with less reliable sunlight and temperature, achieved 6.1% efficiency at turning the energy from the sun into hydrogen fuel. However, indoors, the system achieved 9% efficiency.











