Open Access Government produces compelling and informative news, publications, eBooks, and academic research articles for the public and private sector looking at health, diseases & conditions, workplace, research & innovation, digital transformation, government policy, environment, agriculture, energy, transport and more.
Home 2024
Archives
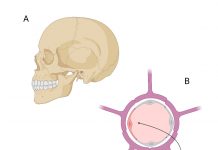
Bridging brain barriers for gene therapy
Reflecting on the challenges in treating brain diseases, this article explores ways to transduce the blood-brain barrier as well as the critical role of tanycytes as a target for gene therapy vectors.
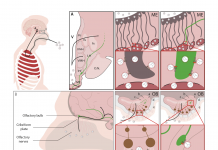
Brain infection by SARS-CoV-2: Lifelong consequences
The WATCH team, founded to elucidate the role played by specialized brain cells called tanycytes in various physiological processes, has been investigating how and where the SARS-CoV-2 virus infects the brain, and some long-term consequences of this neuro-invasion.
The miniNO project: Helping to minimize risks of premature births
Associative mechanisms linking a defective minipuberty to the appearance of mental and non-mental disorders: infantile NO replenishment as a new therapeutic possibility.
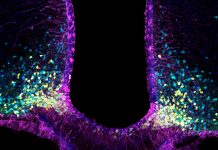
The WATCH project: Tanycytes in health and disease
The WATCH project aims to elucidate how tanycytes mediate physiological processes by acting as gatekeepers between the brain and body, how their dysfunction is involved in various disorders and age-related impairments, and what can be done to prevent or correct these.