Dr Faz Chowdhury, CEO at Nemaura Medical (Nasdaq: NMRD), aims to stem the tide of the onset of diabetes and help to manage and reverse Type 2 diabetes using the world’s first daily-wear non-invasive CGM technology
Nemaura Medical
Nemaura Medical, Inc., founded in 2011, is a UK-based medical technology company that sees itself at the forefront of non-invasive and minimally invasive wearable diagnostic devices. The company hasn’t stopped there, and its technology offering is growing to encompass multiple sensors and AI driven digital platforms, with which Nemaura is positioning itself to become a significant player in the global digital health and wellness revolution.
The company’s programs are a culmination of ten years of research, development, and human trials. We consider our technology to be ground-breaking and represent a paradigm shift in the way that not only people with (Type 2) diabetes can manage their condition but also help over a billion people worldwide track their metabolic health condition and potentially avoid becoming diabetic in the first place. “We believe our device has the potential to significantly change the way people prevent, manage, and even possibly reverse Type 2 diabetes” said Nemaura CEO Dr Faz Chowdhury.
sugarBEAT®
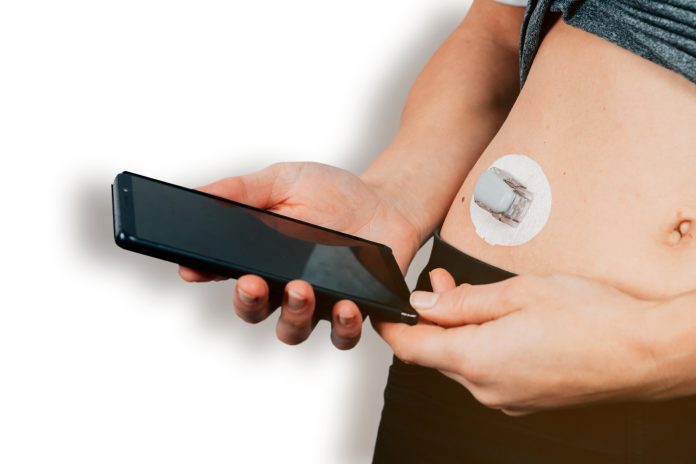
Nemaura’s first product, sugarBEAT® based on their BEAT® wearable sensor platform, a Class IIb Medical device, received C.E. approval in 2019 is the world’s first non-invasive CGM (Continuous Glucose Monitor) where the sensor sits comfortably on the upper arm (not under) the skin. It does not require needles to puncture the skin to insert a sensor. The user is able to monitor glucose levels in real-time without having to scan the device or any other intervention, and the sensor can be worn on any day the user chooses. It is distinguished from other CGMs which require a sensor filament around 1cm long to be inserted in the skin/arm using a large needle, and the wearing of the device continuously for 10-14 days, rendering it inconvenient, costly and invasive, for those in the metabolic health/pre-diabetes stage and indeed for many of those people with Type 2 diabetes who neglect to use finger-prick blood samples for glucose monitoring let alone CGM.
Traditional blood glucose test strips show glucose levels at specific times of the day giving only a point in time measurement which has limited value as the user cannot estimate the glucose levels prior to or in the hours after the test. sugarBEAT® captures readings every five minutes tracking and trending the highs and lows, and monitoring Time in Range (TIR), giving people an overall profile for the day which empowers the user with knowledge of how their glucose levels are fluctuating through the day.
In the UK, the sugarBEAT® technology platform is licensed exclusively to DB Ethitronix, headed by CEO Dr Dallas Burston (MBBS). “We have been able to bring together an easy-to-use digital patient care App, powered by a unique needle-free device for monitoring glucose fluctuations, MySugarWatch®, at a time when there is increased awareness of the risks associated with Type 2 diabetes and diabetes-related COVID-19 risks,” said Dr Dallas Burston. DB Ethitronix recently placed an order for 200,000 sensors with a rolling forecast for a further 2 million sensors over the next 2 years.
Based on the success of the soft launch in the UK, Nemaura is advancing discussions for launches in Germany, Saudi Arabia and UAE. Within Germany, Nemaura are progressing ahead with listing sugarBEAT® for reimbursement on the durable medical catalogue.
Dr. Faz Chowdhury added, “We have been steadily developing a scalable growth plan with a view to building a high-quality product offering in all key global territories. We have now reached a key inflection point and are preparing for a major pivot to accelerate the commercialisation of the sugarBEAT® platform.”
Beating Diabetes in the US one step at a time
Over 463 million people are living with diabetes worldwide, and over $760 Billion was spent in the US alone in 2019 for diabetes-related healthcare expenditure. Knowing this, Nemaura have submitted a Premarket Approval (PMA) for sugarBEAT® to the FDA which is currently in review. While awaiting FDA approval, Nemaura created a program branded ‘BEAT®diabetes’. This incorporates a scientifically proven world-class weight loss program (developed at the Joslin Diabetes Centre, an affiliate of Harvard University), with Nemaura’s glucose monitoring platform.
proBEAT™
The glucose tracking aspect of the BEAT®diabetes program is based on the daily-worn sugarBEAT® non-invasive patch technology which gathers data over the course of the day, profiling the user’s glucose fluctuations relative to lifestyle factors and diet. A report is automatically generated that provides the user an insight into the factors potentially affecting their sugar levels. The aim is to empower users with the right knowledge of factors that could affect their blood glucose levels so they can take action and improve their lives with subtle adjustments to diet and lifestyle, one step at a time.
The BEAT®diabetes program, in conjunction with proBEAT™, is currently being offered in the USA direct to healthcare insurers and employers. Nemaura expects the program to appeal to a broad range of adults encompassing pre-diabetics and people with type 2 diabetes, numbering 88 million and 26 million people respectively, according to the American Diabetes Association.
Whilst the core focus of the company has been a laser sharp attention to providing a solution for the management and prevention of Type 2 diabetes, the company has not stopped there and is planning the launch of a metabolic health program utilising the glucose monitoring patch as the backbone which will be launched direct to the consumer and is expected to appeal to a large percentage of the population, ranging from the health conscious to those with pre-diabetes.
Please note: This is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.