Pushing academic research towards industrial scale through advanced modelling and piloting
At Aalto University’s Department of Energy Technology, Professor Mika Järvinen’s Energy Engineering and Environmental Protection research group is conducting pioneering research on biomass combustion, carbon (CO2) capture and storage (CCS) by mineral carbonation, circulating fluidised bed gasification of waste, and advanced modelling of industrial processes, mainly for energy and metallurgical applications. To foster good industrial collaboration, the group carries out laboratory, pilot and full-scale research to avoid problems in scale-up and provide sustainable and economically feasible solutions for companies.
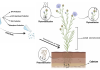
In 2011 the Aalto University Foundation, together with Abo Akademi University and SSAB (formerly Ruukki) Raahe Works, successfully secured a patent for a technique that produces precipitated calcium carbonate (PCC) from alkaline by-products. This CCS approach aims to reduce CO2 emissions by using alkaline industrial waste materials and flue gas rich in CO2 to produce a valuable PCC that is marketable to paper, pharmaceutical or plastics industries, (see the above figure 1). The conventional production of this PCC requires limestone to be mined, transported and subjected to a very energy intensive calcination processes that also emit CO2. However, by substituting limestone with industrial Ca-rich wastes (e.g. steel slag) our method reduces waste, saves energy and reduces CO2 emissions.
Almost 1500 Mt of steel is manufactured globally every year producing 2700 Mt CO2 corresponding 6.7% of anthropogenic emissions. In addition to the direct and indirect emissions of CO2, the production of steel also annually generates about 400 Mt of a solid by-product known as slag. Slag is formed from species in pig iron such as Si, Mn, Mg, some Fe and also valuable Cr that are oxidised during steelmaking. One major component in slag formation is CaO that is fed to the top of the slag to remove silicon and sulphur and improve flowability. CaO is produced in the lime kiln by heating the limestone up to 1100 °C.
There are several different types of slag produced at different stages of the steelmaking process. Basic oxygen steel converter slag is very well suited for a Slag2PCC process as it contains a large amount of free calcium that can be effectively extracted, with annual slag availability being 135 Mt. Steelmaking slag finds applications in road construction as well as the cement industry, however, about 13% is unusable for these purposes and is generally landfilled. Figure 2 presents the simplified principle of our Slag2PCC process implemented to steelmaking.
Steelmaking requires calcium oxide CaO that is produced from CaCO3. In our process, a water solution of an ammonium salt is used to extract calcium from the steelmaking slag. The resulting Ca-rich solution is bubbled with CO2 gas which reacts to form PCC, precipitated calcium carbonate. The quality of synthetic PCC generally surpasses that of even the highest quality natural ground PCC as the process can be tailored and controlled to produce a wide variety of PCC products with very high purity and different crystal properties. For this reason, PCC can be sold at a higher price. The global consumption of PCC increased from 10 Mt in 2004 to 14 Mt in 2011 and is expected to continue to grow. There are many requirements for the PCC properties including particle size, purity, brightness and crystal morphology, and these vary depending on the application.
Current research challenges and main developments
There could be a significant surplus of PCC available if all steel converter slag production in the world would be utilised by our process. As one example, our synthetic PCC could partly replace the limestone used in the production of steel, reducing the need for mining. PCC could be sold at a higher price as a commodity chemical to several other industries. We are also able to bind most CO2 emissions, 30 Mt, related to calcium treatment in the steel mill. If the lime kiln is heated by biofuels, the process would be mostly CO2 neutral. The global annual emissions from the blast furnace in the iron making are in the order of 2600 Mt, being far too large for Slag2PCC process to be applicable. The slag from the blast furnace is also not suitable for our process and is already mainly used in the cement industry. The biggest benefit from our process is in the reduced need of virgin limestone required for steel converters.
The feasibility of PCC production from steelmaking slag has been successfully demonstrated by our pilot plant, launched in 2014, both on batch and continuous modes. We can successfully produce high-quality PCC of rhombohedral calcite and aragonite of various sizes. We have recently published results showing that by applying ultrasonic extraction, extraction efficiency can be significantly increased (Said et al. Enhancement of calcium dissolution from steel slag by ultrasound, Chemical Engineering and
Processing 89 (2015) 1–8). We are also currently working on developing alternative PCC products based on the particular advantages of the Slag2PCC process for potential high-value niche applications. Recovery of the ammonium salt solvent and effectiveness of the filtering are still critical aspects of the process and how to best achieve this at minimal energy cost is a major challenge.
Mika Järvinen and Arshe Said
Aalto University
Department of Energy Technology
Tel: +358 50 4142593
mika.jarvinen@aalto.fi