Dr Olga V Volpert’s work on sunlight-induced carcinogenesis at the MD Anderson Cancer Center is explored here, including why angiogenesis is critically important in skin cancer progression
Sunlight-induced carcinogenesis has become a concern since the 1980s when the depletion of the ozone layer was first noticed. Long-term exposure to ultraviolet B (UVB) component of sunlight is a risk factor for both non-melanoma and melanoma skin cancers. The incidence of skin cancers is increasing, with 2-3 million non-melanoma skin cancers and 132,000 melanoma cases occurring globally each year. Skin is sensitive to photodamage even after short-term exposures to UVB.
As with other cancers, angiogenesis is critically important in skin cancer progression and UVB-induced vascular changes in the skin have been attributed to increased production of pro-angiogenic cytokines and chemokines, such as vascular endothelial growth factor (VEGF), cyclooxygenase (Cox)-2, basic fibroblast growth factor (bFGF) and interleukin-8 (IL-8).
Thrombospondin-1 (TSP1), a large glycoprotein abundant in healthy adult tissues is a major inhibitor of angiogenesis. Loss of TSP1 has been implicated in the progression of many cancers including breast and colon carcinomas and skin cancers. In the skin, TSP1 produced by keratinocytes in the epidermis acts as a gatekeeper of the vascular changes associated with and required for cancer progression.
Therefore, TSP1 seems an obvious candidate for prevention or treatment of cancer. However, a very large protein with complex functions is difficult to manufacture for clinical purposes. What if there was a compound that impedes subcutaneous angiogenesis by keeping normal, high TSP1 levels in the skin? There may be such a compound coming from a medicinal or culinary plant, chamomile or parsley.
Chamomile flowers are known since antiquity, for their healing properties. Apigenin, an active ingredient found in chamomile, has potent chemopreventive properties against UVB-induced skin cancer. In addition to its cytotoxic effects, potentially useful for arresting the growth of rapidly multiplying tumour cells, apigenin inhibits angiogenesis.
Using cultures of human and mouse keratinocytes, as well as mouse models, Dr Volpert and colleagues carried out studies confirming the anti-angiogenic effects of apigenin and showed that it can reverse TSP1 loss in the UVB-exposed skin. This is the first known report of apigenin directly controlling endogenous anti-angiogenic protein. Treatment with apigenin, before or after UVB irradiation, restored TSP1 levels in cultured cells and in the skin.
They also found that, like apigenin, an active TSP1 peptide inhibited the production of Cox-2 and VEGF and reduced UVB-induced cancer-related vascular effects, angiogenesis and epidermal thickening. These studies strongly suggest the benefits of apigenin for therapy or prevention of skin cancer and provide mechanistic insights into its protective action.

Angiogenesis inhibitor alters the immune landscape in skin
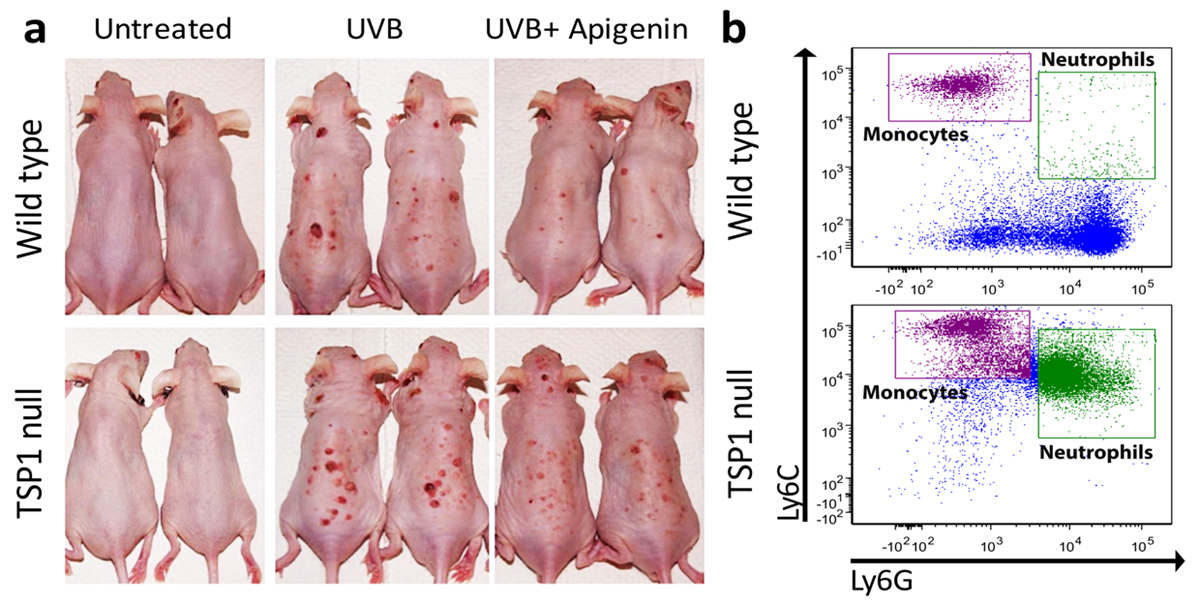
Further studies by the Volpert group, indeed, showed that apigenin efficiently inhibits the UVB-induced skin carcinogenesis in wild-type mice but loses its anti-cancer effect, in which TSP1 gene is disrupted (TSP1 null) (Fig. 1a). Surprisingly, the most dramatic change in TSP1 null mice is in the inflammatory component of a tumour. It appears that TSP1 interferes with the UVB-induced production of inflammatory cytokines IL-6 and IL-12 and that in mice null for TSP1 far more inflammatory cells – neutrophils and inflammatory monocytes are recruited from bone marrow to skin where they produce growth factors to support cancer progression (Fig. 1b).
Angiogenesis Inhibitor is transferred by exosomes to bolster innate immune surveillance
Exosomes are tiny physiological vesicles (50-150 nanometres in diameter) formed through the endosomal pathway and released by all cells in the body. Exosomes and carried in biological fluids, like serum and urine. Studies of the past decade have identified exosomes as natural vehicles assisting communications between cells and distant tissues.
These natural nanovesicles bioactive molecules to the recipient cells and change their properties or behaviour. Most studies show that exosomes released by cancer cells promote metastasis by creating permissive environments at the sites of their arrival (metastatic niches).
One of the major effects of cancer exosomes is immunosuppression. By transferring immunosuppressive cytokines and immune checkpoint inhibitors, cancer exosomes can incapacitate natural killer and cytotoxic T cells. This deficient host immune response serves to protect disseminating cancer cells and facilitate metastasis.
In a recent study, Dr Volpert and colleagues determined that cancer cells can also activate early immune surveillance. Working with melanoma as a model, they have demonstrated that at an early stage, melanoma cells alert the immune system of the host to the presence of metastasis by sending out exosomes, which activate highly specialised cells called patrolling monocytes.
In the absence of cancer, patrolling monocytes constantly scan the blood vessel to seek out and eliminate damaged or dying cells. In case of metastatic cancer, the patrolling monocytes can detect and destroy lurking cancer cells. The Volpert group showed that one of such exosome-associated activators of patrolling monocytes is a known angiogenesis inhibitor, pigment epithelium-derived factor (PEDF).
Once activated with PEDF-containing exosomes, patrolling monocytes kill and engulf cancer cells on their own or recruit other cancer-killing immune cells, called natural killer cells. This is yet another case where anti-angiogenic protein ‘doubles’ as an activator of immune response and stops the spreading of cancer cells to the distant organs (Fig. 2).
Please note: this is a commercial profile
Dr Olga V Volpert
Associate Professor
MD Anderson Cancer Center
Tel: +1 832 750 1521
https://mdanderson.influuent.utsystem.edu/en/organisations/cancer-biology