Karen Taylor, the Director at Deloitte Centre for Health Solutions looks at 4P medicine and its role in healthcare diagnostics
Diagnostics play a pivotal role across the entire healthcare continuum from screening, detection and prognosis to patient stratification and condition monitoring. Diagnostic tests affect most healthcare decisions, supporting clinicians to make more accurate diagnoses and prescribing the right treatments. Earlier access to diagnostic tests can help avoid adverse health outcomes and the higher cost of late-stage or unnecessary treatments.
Diagnostics can also enable a shift from reactive, episodic treatment, to predictive, preventative, personalised and participatory 4P medicine and care.
This article provides an overview of our research findings, exploring what the future of diagnostics in Europe might look like. They are derived from an extensive literature review, semi-structured interviews with 40 key stakeholders, survey responses from 250 diagnostics companies, survey responses from 751 front-line clinical staff (clinicians) and insights from Deloitte colleagues.
Why diagnostic services in Europe need to change
The diagnostics industry comprises a wide range of diagnostic devices and tests, from high value relatively low use imaging tests to low-cost, high-use in vitro diagnostic (IVD) tests. However, IVDs, which influence over 70% of medical decisions account for only 0.8% of healthcare spending.
Prior to the COVID-19 pandemic, demand for diagnostic services (imaging, pathology, endoscopy and genomics) was increasing at a faster rate than capacity. Moreover, the overall costs of delivering healthcare were increasing.
The pandemic catalysed innovation in diagnostics across the health ecosystem, accelerating adoption and shifting the location of testing from hospitals and centralised laboratories to closer to the patient (57% of surveyed clinicians recognised this shift and 77% of companies said their products were part of this move).
Turning challenges in product development and adoption into enablers
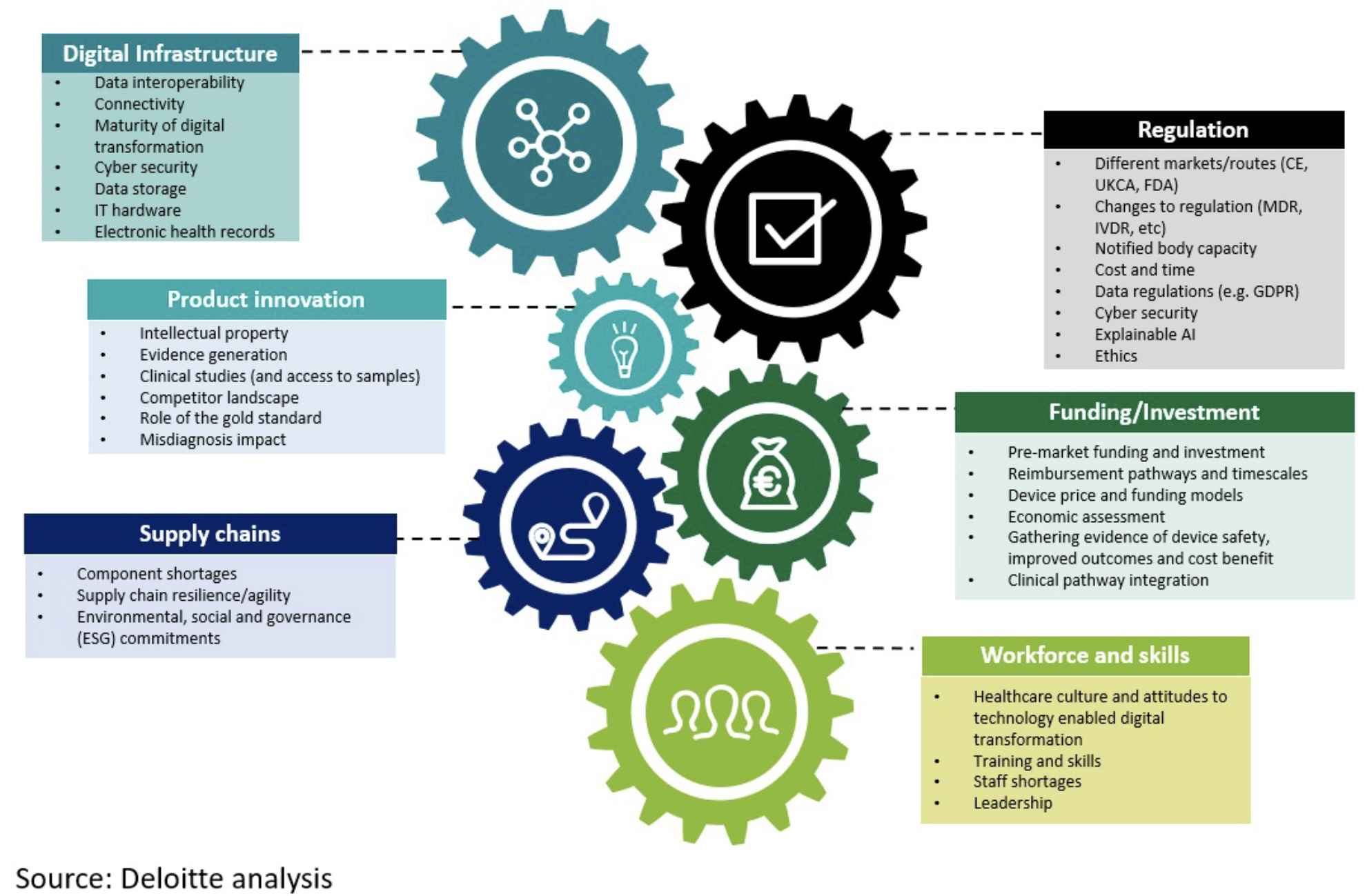
We identified six overarching challenges facing the development and adoption of new products which, if tackled effectively, could become enablers for the future of diagnostics (Figure).
Digital infrastructure: 51% of diagnostics companies identified healthcare’s digital infrastructure especially inter-operability and connectivity, as their top challenge in bringing a new diagnostic to market. They also identified data sharing as crucial to improving diagnostic services.
Regulation: Companies face many challenges due to the EU’s May 2021 medical devices and May 2022 IVD regulations; including more stringent evidence requirements and lack of capacity causing concerns over the time taken to perform conformity assessments. Interviewees suggested that regulators should create target product profiles and clear indications of requirements to enhance transparency and guide product development conformity.
Product innovation: Innovation pathways need to align with clinical needs and be co-developed with end users. Maintaining the security of diagnostic data is also vital, consequently ‘security by design’ should be embedded from the start. However, concerns over the new regulations were making Europe a less attractive market for product launches.
Funding and investment: The new regulations appear to be undermining access to innovation funding and increasing the costs of meeting the more exacting evidence requirements. A third of diagnostics companies considered themselves ‘not very well’ or ‘not at all’ prepared for obtaining sufficient funding to develop and launch products. Moreover, each European country has its own reimbursement policies for clinical evidence. A Europe-wide innovation ecosystem is needed to support manufacturers in the development and launch of products.
Supply chain: Supply chain issues affecting European manufacturers include the post-pandemic recovery, Brexit, and geopolitical turbulence. Our interviewees have developed multiple strategies to safeguard their supply chains, including higher levels of inventory and access to multiple sources for materials and components. However, new regulatory requirements for product traceability and global ambitions for achieving net zero, are expected to have major supply chain implications.
Workforce: Across Europe healthcare systems are facing significant staff shortages. Adoption of new diagnostic technologies can help improve efficiency and enable clinicians to make more accurate and timely decisions. Clinicians felt a lack of workforce training and skills in new technologies was a core barrier to technology adoption. While healthcare systems should provide such training, companies have a responsibility to ensure they provide appropriate learning materials, on-demand support, and on-site training.
A new diagnostic paradigm enabling 4P medicine
Disruptive technologies, advances in science and analytics are combining to transform the way we prevent, diagnose and treat disease, with the industry now entering the fourth industrial revolution. Digitalisation, robotisation and automation are giving rise to smart laboratories and smart imaging systems to handle demand at greater speed, accuracy and lower cost.
Our clinicians identified telehealth, robotics, biosensors, and AI as the technologies already starting to enhance diagnosis, but are less certain about others. Our research identified many disruptive technologies being adopted in pockets, but which, if adopted at scale, will enable the future of 4P medicine.
Conclusions for the healthcare system
Healthcare systems are in a period of transition, moving from inefficient, largely reactive, and episodic, models of care to 4P care models. Diagnostic companies can capitalise on the opportunities presented to become insightful partners in clinical pathways, helping to diagnose patients earlier and faster, identify more appropriate treatments and deliver more patient-centric, value-based care.
Changing the dial to increase the priority given to improving access to diagnostics has the potential to improve equity and health improve outcomes for everyone. This requires an industry-wide effort to help all stakeholders appreciate the role of innovative diagnostics within healthcare, and a concerted effort to translate innovation into improved outcomes.

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.