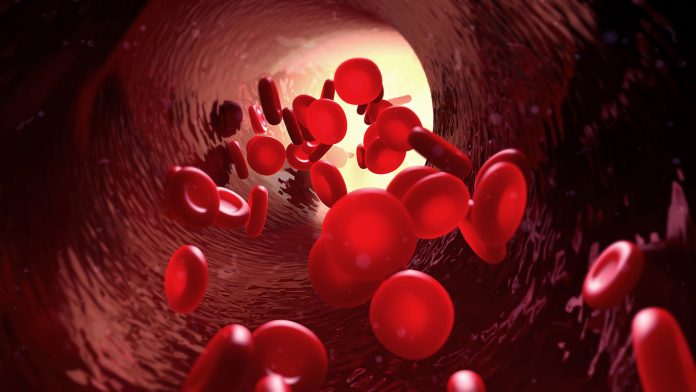
Scientists from Boston University (BU) and the Wyss Institute at Harvard University have developed a new method called ESCAPE, which is engineered sacrificial capillary pumps for evacuation that could improve how tissue is fabricated and designed
The team, supported by the U.S. National Science Foundation (NSF), have used gallium, a metal that can be moulded at room temperature, to create moulds for soft biological materials. To create 3D-printed blood vessels.
This new technique could help create tissue structures that imitate the complexity and scale of the ones found in human organs, which is a step towards advancing regenerative medicine and prosthetic development.
3D-printed blood vessels and live cells
Traditional artificial tissue creation methods have struggled to replicate the small and large structures needed for functional organs. 3D printing, which has changed industries from aerospace to healthcare, is commonly used in biomedical applications, especially for creating prosthetic devices.
Printing with living cells remains a significant challenge due to the delicate nature of tissues and the need for precise control over small-scale features such as blood vessels. While some methods have successfully created either large or small structures, they have not been able to create both simultaneously within a single scaffold, limiting their usefulness for tissue engineering.
ESCAPE overcame this limitation using gallium-based moulds; the researchers can produce multiscalar structures—combining both millimeter-scale and micrometer-scale features—within the same tissue scaffold.
This is key for creating tissues like blood vessels, which require large arteries and tiny capillaries to function correctly. With ESCAPE, the team successfully created branched vascular networks in collagen, spanning from millimeter-scale arterioles down to microvasculature just a few micrometers wide. These types of vascular structures are difficult to fabricate and essential for tissue survival in engineered organs.
ESCAPE changing medical landscape
The key advantage of the ESCAPE technique is that it decouples the process of creating the structure from the material being used. By using gallium, a metal that can be moulded at low temperatures, researchers can form detailed scaffolds without damaging the sensitive biological materials that make up the tissue.
Once the gallium mould is created, it is removed, leaving behind a structure that can then be filled with living cells to form functional tissue. This approach opens up new possibilities for creating more complex tissues that better mimic the natural environment of the human body.
The researchers’ early focus has been on vascular tissues, which are especially complex due to the many sizes of vessels required to create a functional network. Vascular structures must include large arteries for nutrient transport and tiny capillaries that exchange gases and waste products.
The ESCAPE method has demonstrated success in creating these networks with a high level of detail, including features such as dead ends and areas where fluid flow is possible, which are important for modeling both healthy and diseased tissue.
The implications of this research are far-reaching. In addition to creating vascular networks, ESCAPE could eventually be used to build other complex tissue architectures, such as those found in organs like the heart, liver, or kidneys. The ability to construct tissues with multiscale features could significantly improve the development of organ patches for patients needing transplants or treatments for organ failure.
As technology advances, computational modeling will be used to refine and test these structures further, expanding the types of materials that can be reproduced with this method. With continued support from organisations like NSF, the potential for ESCAPE to revolutionise tissue engineering grows, offering new possibilities for regenerative medicine and providing critical insights into how tissues form and function in the body.