Dr Roger J. Young, Professor at Saba University School of Medicine, walks us through Brittle Bone Disease, otherwise known as Osteogenesis Imperfecta Type I
Brittle Bone Disease, otherwise known as Osteogenesis Imperfecta Type I, is a manifestation of a range of pathologies originating from the malfunction or malformation of the protein collagen. As devastating as this disease can be, it is one of the milder pathologies associated with the malfunction of this protein, with variants ranging from asymptomatic to incompatibility with life.
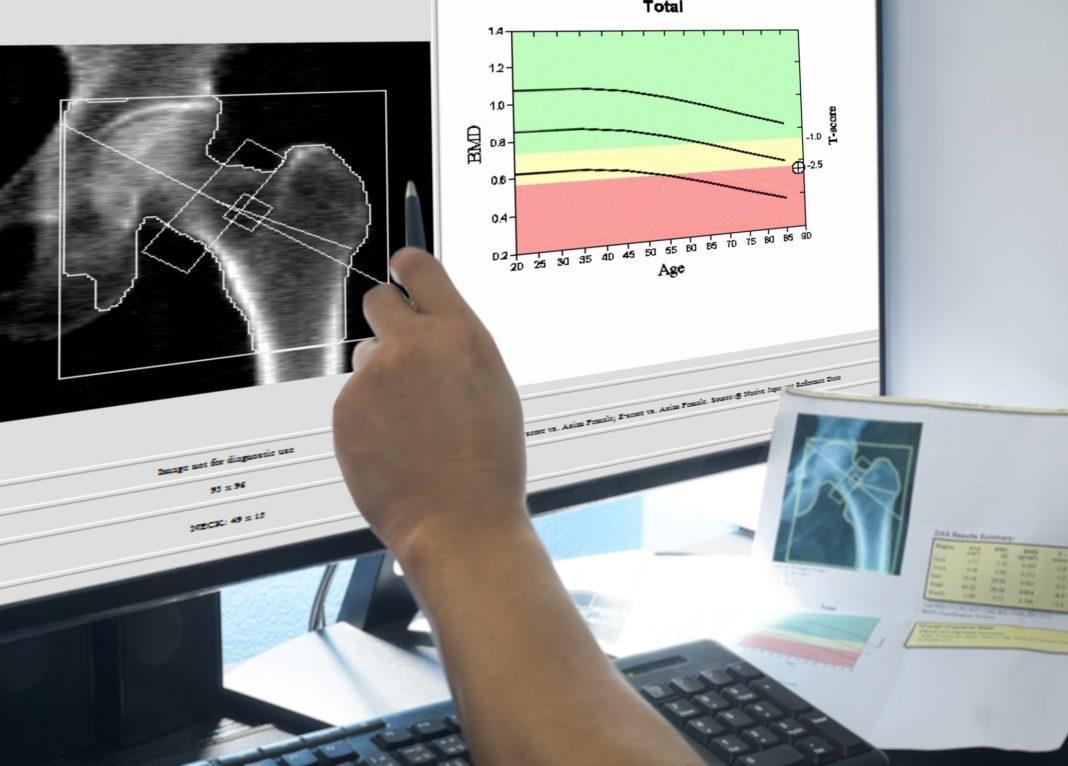
Brittle Bone Disease has a prevalence in the UK of about one in fifteen thousand with similar frequencies being reported in other locations and individuals with this disease display increased susceptibility to bone fractures along with decreased bone density. Diagnoses may frequently be post-natal with multiple bone fractures occurring during delivery of the affected individual. Other symptoms include blue sclera of the eyes where the reduction in collagen allows the sclera tissue to be translucent, deafness due to the malformation of the middle ear bones, and dental abnormalities. Bone density scans in older children are useful in diagnosis as well as X-rays to visualise fractures that have previously occurred.
Collagen exists as several subtypes and is the most abundant protein in mammalian systems with Type I collagen being the most prevalent form in the body where it performs many roles as the main protein in the extracellular membrane in skin, bones, tendons, heart and other organs. Given its near ubiquitous nature, it is no wonder that an error in collagen function can have far reaching effects throughout the body.
Collagen synthesis in fibroblast cells
To understand the disease, it helps to summarise the process of collagen synthesis in fibroblast cells found in connective tissue. Transcription of the COL1A1 and COL1A2 genes and subsequent translation of their mRNA products creates a polypeptide repeat of glycine, hydroxylysine & hydroxyproline which assumes a helical structure. While still in the cytosol, three of these polypeptide chains (two from the COL1A1 gene, and one from the COL1A2 gene) are intertwined as a triple helix and then secreted into the extracellular space.
Here, the ends of the triple helix are trimmed, and the remaining structure crosslinked to other triple helices of collagen. In a manner analogous to forming a strong thick rope from a large number of intertwined and twisted thin fibers, the structurally functional collagen protein is then able to perform its many molecular roles. Any alteration in this enzymatically and spatially complex process will alter the end product such that it has different and maybe deficient properties compared to the unaltered product. While the most commonly mutated genes involved in collagen synthesis are the alleles mentioned above, given the complexity of the synthesis process there are more than a dozen other genes that when mutated are also implicated in Brittle Bone Disease.
The systemic distribution of collagen and its essential role in many different organs is a major challenge in the treatment of Brittle Bone Disease. While relatively new molecular techniques such as CRISPR can target individual nucleotide mutations within a cell and the use of assorted viral vectors can target specific tissues, the specific alteration of a genetic mutation possibly unique to that individual, throughout the majority of cells in the body provides a remarkable goal.
Noninvasive prenatal testing
The practice of noninvasive prenatal testing is well established. Obtaining fetal cells via a simple blood draw provides minimal risk to the mother and enables a full genome screen of the fetus to determine the presence of mutations in alleles involved in collagen synthesis. Knowledge of the risk to the fetus of Brittle Bone Disease can allow the parents to prepare for such an eventuality and enable physicians to choose safer options for delivery to minimise bone fractures. Brittle Bone Disease is a dominantly inherited trait, and most cases have a familial history, therefore education of prospective parents combined with prenatal testing will provide early information and allow parents to choose their course of action.
Treatment and research for Brittle Bone Disease
While Brittle Bone Disease can be treated with bisphosphonate to reduce bone loss along with prophylactic vitamin D, it has also been shown that all- trans retinoic acid (from Vitamin A) can stimulate collagen production in aged and photodamaged skin, therefore diet modification could be a pre-emptive step into reducing the effects of this disease.
While research into curing Brittle Bone Disease alone possesses many challenges, doing so also improves our understanding of collagen synthesis and function in many of the other roles that it plays in our tissues. With an increasing global percentage of elderly individuals, collagen deficient age-related maladies are on the increase as octogenarians not only produce less collagen than their younger peers, but also fail to successfully crosslink collagen fibers leading to a variety of collagen molecular modifications. These age- related maladaptations may be manifested as a variety of pathologies including chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis.
The malformation of collagen then, may result in a variety of pathologies that present in a multi-system manner, and as such a direct targeted approach to curing Brittle Bone Disease will be challenging with behavioral and dietary modifications providing the most immediate benefit. Rapid advances in genetic testing techniques however now provide parents and researchers alike the opportunities to study individual genetic changes and prepare in vitro or in utero treatments as appropriate. With collagen modifications affecting multiple pathologies from lung and heart disease to cancer, continued research into Brittle Bone Disease will also yield advances in these areas and provide benefits to others who suffer from collagen related ailments.