Andrea Varrone studies the importance of molecular imaging to provide personalised medicine and improve care for patients with neurodegenerative disorders
Neurodegenerative disorders represent a heavy burden for the society at a global level. In 2015 there were 46 million cases of Alzheimer’s disease (AD) and other dementias and approximately 6 million cases of Parkinson’s disease (PD) worldwide1. From 1990 to 2015 the prevalence of both disorders has more than doubled and the burden for the society has increased with increasing age1. Tools for personalised medicine and improved care of patients with neurodegenerative disorders are needed, including disease biomarkers for early diagnosis, patient stratification and assessment of treatment efficacy.
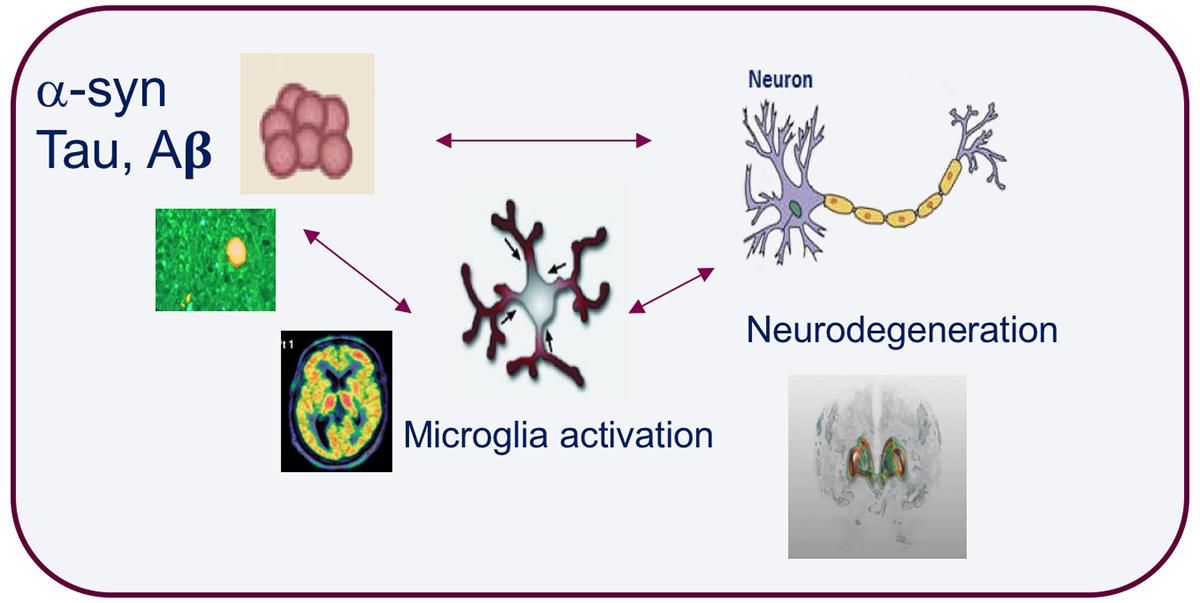
The development of brain imaging tools that target specific molecules and disease processes is key to understand the pathological changes associated with neurodegenerative disorders. Accumulation of misfolded proteins such as amyloid-beta, tau and alpha-synuclein, along with synaptic dysfunction, synaptic loss and neuroinflammation are pathological processes common to major neurodegenerative disorders. Molecular imaging with positron emission tomography (PET) can facilitate the development of markers that enable to study these processes in vivo (see Figure).
Molecular imaging markers can be used as tools for early diagnosis and patient stratification and as endpoints to assess the effects of disease-modifying treatments, thus supporting drug development programs.
Imaging of amyloid-beta and tau, present and future
At present, several PET agents are available to image amyloid plaques in prodromal or symptomatic AD. Tau accumulation also plays a key role in the natural course of the disease. Tau imaging agents have enabled to examine in vivo the distribution of tau pathology according to Braak staging, but also the relationship between tau accumulation and neurodegeneration and between tau and cognition2. Most of the first generation radioligands, however, showed some limitations related to the presence of off-target binding3, which limits their wide application in AD and non-AD tauopathies, such as progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS).
New tau imaging agents with less off-target binding are now available and show promising results in detecting tau pathology in vivo in AD and PSP. The thorough in vivo characterisation of new tau imaging agents including quantification with kinetic analysis and compartmental modelling will be key for their validation as clinical imaging agents in AD, PSP and CBS, as well as potential biomarkers for clinical trials using anti-tau immunotherapies.
Alpha-synuclein – the molecular imaging “unicorn”
The development of an agent able to image alpha-synuclein in PD and other synucleinopathies such as multiple system atrophy and Lewy body dementia is an undisputable unmet need. Although several efforts have been made, a PET imaging agent for Lewy body pathology is yet not available. Such PET agent is of high need considering also potential therapeutic approaches to target alpha-synuclein pathology that are under development4.
Synaptopathy – imaging of synaptic density
Alpha-synuclein pathology in PD is associated with synaptic dysfunction and degeneration5. The concept of synaptopathy is a broad term that indicates changes induced by different types of pathological mechanisms leading to synaptic dysfunction. Synaptopathy is characteristic of neurodegenerative disorders, but also of schizophrenia and autism spectrum disorders. The availability of PET radioligands for imaging the synaptic vesicular glycoprotein 2A (SV2A) enables to measure synaptic density in vivo. Reduced SV2A availability in hippocampus of AD patients6 was recently reported and studies in PD and schizophrenia are ongoing. SV2A is a general marker of synaptic density, whereas SV2C is specifically expressed in the nigrostriatal system and found to be altered in PD7.
Therefore, a PET radioligand for SV2C will be more specific to study synaptopathy in PD. At present, the most straightforward approach to examine the synaptic integrity of dopaminergic terminals in PD is dopamine transporter (DAT) imaging. Studies have shown that while there is a profound loss of DAT in the dopaminergic terminals, in early PD the DAT is relatively preserved in the axons and the cell bodies8, supporting the view that synaptic loss is the earliest pathological event and that synaptopathy eventually leads to a retrograde or “dying back” like type of degeneration5.
Neuroinflammation
Microglia activation and neuroinflammation have been of major interest for the neuroimaging community and considerable efforts have been made to find suitable PET imaging agents. The 18-kD translocator protein (TSPO) has been for decades the major target to image neuroinflammation in vivo. Initial studies with the first TSPO PET agent [11C]PK11195 reported increase binding to TSPO in AD, PD, as well as other parkinsonian disorders.
Subsequent studies with second-generation TSPO radioligands have reported different results, not necessarily replicating initial findings observed with [11C]PK11195. TSPO is not selective for microglia as it is also expressed on astrocytes. Therefore, there is a need for new targets that can be more selective for imaging microglia activation. Work on some of these new targets as the purinergic receptors P2X7 and P2Y12 is ongoing and further development is needed to identify better agents for imaging neuroinflammation in neurodegenerative disorders.
References
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the {Global} {Burden} of {Disease} {Study} 2015. Lancet. Neurol. (2017). doi:10.1016/S1474-4422(17)30299-5
- Bejanin, A. et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer-s disease. Brain (2017). doi:10.1093/brain/awx243
- Okamura, N. et al. The development and validation of tau PET tracers: current status and future directions. Clinical and Translational Imaging (2018). doi:10.1007/s40336-018-0290-y
- Brundin, P., Dave, K. D. & Kordower, J. H. Therapeutic approaches to target alpha-synuclein pathology. Experimental Neurology (2017). doi:10.1016/j.expneurol.2017.10.003
- Bridi, J. C. & Hirth, F. Mechanisms of α-Synuclein induced synaptopathy in parkinson’s disease. Frontiers in Neuroscience (2018). doi:10.3389/fnins.2018.00080
- Chen, M.-K. et al. Assessing Synaptic Density in Alzheimer Disease With Synaptic Vesicle Glycoprotein 2A Positron Emission Tomographic Imaging. JAMA Neurol. (2018). doi:10.1001/jamaneurol.2018.1836
- Dunn, A. R. et al. Synaptic vesicle glycoprotein 2C (SV2C) modulates dopamine release and is disrupted in Parkinson disease. Proc. Natl. Acad. Sci. (2017). doi:10.1073/pnas.1616892114
- Fazio, P. et al. Nigrostriatal dopamine transporter availability in early Parkinson’s disease. Mov. Disord. (2018). doi:10.1002/mds.27316
Please note: this is a commercial profile
Andrea Varrone, MD, PhD
Associate Professor/Senior Lecturer
in Nuclear Medicine
Karolinska Institutet
Department of Clinical Neuroscience
Centre for Psychiatry Research
Tel: +46 70 744 27 28