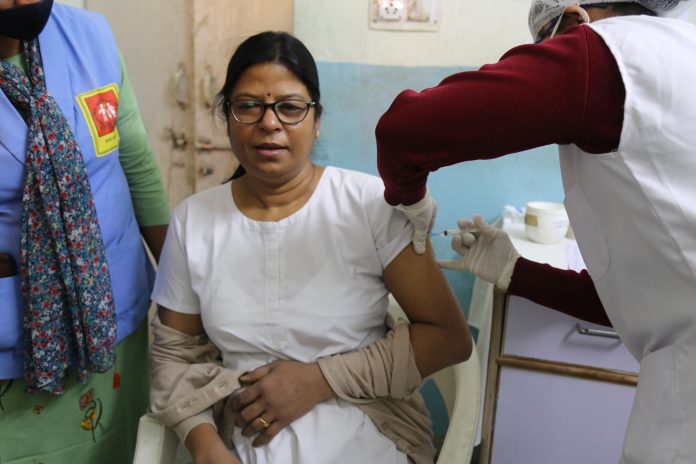
Rachel Thrasher, Research Fellow at the Global Development Policy Center in Boston, explains how the TRIPS COVID-19 waiver rejected by the WTO could ramp up global vaccine production
At the informal meeting of the Council for the Agreement of Trade-Related Aspects of Intellectual Property (TRIPS) on February 4, the United States, together with the European Union, United Kingdom, Japan and Australia continued to block the initiative to waive certain World Trade Organization (WTO) provisions that potentially constrain manufacture and disbursal of COVID-19 medicines, diagnostics, medical equipment, and vaccines.
What is the TRIPS COVID-19 waiver?
This narrow waiver, proposed initially by South Africa and India, would temporarily waive patent rights over these products to facilitate increased production volume and more widespread manufacturing worldwide. Nevertheless, while the US and the EU push for more discussion about the facts of the current situation, South Africa, India, and others are seeking to negotiate the text of the proposed waiver. At the moment, the talks are at an impasse.
At the moment, the talks are at an impasse.
But evidence is mounting that signing the TRIPS waiver would not only be good for the current supporters of the initiative, but for the whole world, and maybe especially for the developed countries who are currently opposed to it. The financial costs to all countries during the pandemic goes far beyond paying for the research and development, treatments and vaccines to manage COVID-19 cases. Economic impacts will be felt across the global economy through supply chain disruptions rooted in growing inequality within and between countries, likely costing around $9.2 trillion dollars, half of which would be borne by a handful of developed economies.
Economic impacts […] likely costing around $9.2 trillion dollars
The projected timeline for vaccinations exacerbates the financial costs. Initial predictions for vaccine rollout all over the world have proven optimistic at best and current projections suggest that many will have to wait at least three, and up to seven, years for substantial global immunity through vaccines, leaving low-income countries hopelessly behind.

© Mangala Sardar
The lack of manufacturing capacity by drugmakers
One of the main reasons the vaccines have not become as widely available as initially hoped is the lack of production capacity by key firms. For obvious reasons, a small handful of corporations cannot produce enough vaccines for the whole world population. Producing enough will depend heavily on licensing and transferring technology to more manufacturers. This reality is highlighted by a recent case in which a vaccine innovator company (Inovio) sued its own contracted biologics manufacturer (VGXI) because they refused to release their own trade secrets to other potential producers in order to ramp up capacity. These same supply capacity issues afflict other more well-known companies as well – including Novavax and Moderna.
Pharmaceutical companies would prefer to rely on voluntary licensing agreements (VLAs) to increase production. These VLAs allow the patent holder to control who is producing their patented good and where they are able to sell the product. Gilead’s VLA to produce remdesivir is the most widely known example of such a process. While initially applauded for increasing access and to a potentially life-saving treatment for COVID-19 at affordable prices, further research showed that the agreement excluded 70 countries who would have to purchase the drug at the monopoly price. Given that cautionary tale, it is unlikely that VLAs would be enough to ensure widespread access.
The rigid reality of the TRIPS Agreement
Many countries who push back against a TRIPS waiver suggest that the TRIPS Agreement is already flexible in its allowance of compulsory licensing to facilitate generic manufacture of patented vaccines.
The agreement allows member states to authorise compulsory licenses (CLs) under their own domestic law in cases of extreme urgency, as long as the scope and duration of the license is narrowly circumscribed. In ordinary circumstances, countries can impose a CL if they are unable to negotiate a voluntary license within a reasonable period of time. In both cases, the innovator is due “adequate remuneration” (Art. 31). Certainly, there has never been a case of extreme urgency like this one, and WTO members theoretically may have recourse to this provision.
However, previous CLs issued by member states have met with both public and private opposition. The United States has repeatedly put pressure on India for its CL on an expensive cancer drug, claiming that India is “diluting” intellectual property rights and violating the TRIPS Agreement. Private pharmaceutical companies and U.S. lawmakers have even taken action to threaten sanctions against India through its Special 301 Report, a trade watch-list of sorts.
Colombia faced similar backlash when they took the first steps toward issuing a CL for a leukemia treatment – Glivec. Both the Swiss government and Novartis, the patent holder, argued forcefully that CLs are “tantamount to expropriation” – code for exercising a sort of eminent domain through regulation. More recently, Malaysia attempted to use a CL to increase affordability of a Hepatitis C medication and once more the United States, together with its pharmaceutical industry, threatened to wield the power of sanctions through a Special 301 Report. As a result of these and other instances, countries have, understandably, been reluctant to develop more flexible domestic CL policies and are certainly out of practice in using them.
A TRIPS COVID-19 waiver opens up global production
Given the challenges of imposing compulsory licenses and the limits of voluntary ones, the TRIPS waiver offers another way for vaccine producers around the world to ramp up global production without the risks of contending with domestic and international IP disputes.
the TRIPS waiver offers another way for vaccine producers around the world to ramp up global production
Additionally, the countries opposing the TRIPS waiver make two other key and yet contradictory, arguments.
In the first place, they argue, intellectual property protection is what made these vaccines possible to begin with – undermining those rights, then could undercut the potential for future lifesaving products. The protection of intellectual property is certainly aimed at increasing innovation, and some studies have shown that innovation does increase with greater protection. At the same time, other research suggests that strong IP protection could actually discourage subsequent innovation.
Even without disregarding the valuable role of intellectual property protection, however, the TRIPS waiver would not dismantle our current system of innovation incentives. Rather it is a narrow, time-limited waiver aimed only at facilitating global access to COVID-19 related products. Most of the vaccine developers have already received ample government support for the research and development stage – diminishing the need for patent monopolies (which are supposed to make up for large up-front capital expenditure).

The second argument put forward by opponents of the TRIPS waiver points out that intellectual property rights are not the real bottleneck preventing more rapid global production, at least in the case of vaccines. Rather, the manufacturing capacity of most of the world’s countries is simply not advanced enough to make these types of vaccines.
But this argument seems to run up against the vein of the previous contention – if intellectual property rights are not the issue, if no vaccine manufacturers are going to be able to ramp up production to make any kind of real difference in distribution, then there’s no point in being concerned about temporarily waiving those rights. The current producers will still effectively benefit from their patent monopolies.
The current producers will still effectively benefit from their patent monopolies.
On the other hand, there is growing evidence that perhaps qualified producers around the world stand ready to contribute to the production of more vaccines. Despite an unknown timeline, there is a real possibility that the TRIPS waiver may make it possible for a huge increase in vaccine production, not to mention the production of other COVID-19 treatments and equipment.
When could the TRIPS COVID-19 waiver happen?
Looking toward the next scheduled meeting of the TRIPS Council on February 25, the rationale behind protecting the profits of large pharmaceutical companies seems to be weakening. Signing the TRIPS waiver for COVID-19-related products, treatments and vaccines is the right thing to do, and everyone will benefit from it.
Rachel Thrasher is a researcher with the Boston University Global Development Policy Center. She works on policy issues related to trade and investment agreements, trade law and development, economic relations between developing countries, and multilateral environmental agreements. She is the author of Constraining Development: The Shrinking of Policy Space in the International Trade Regime (Anthem, forthcoming, July 2021).