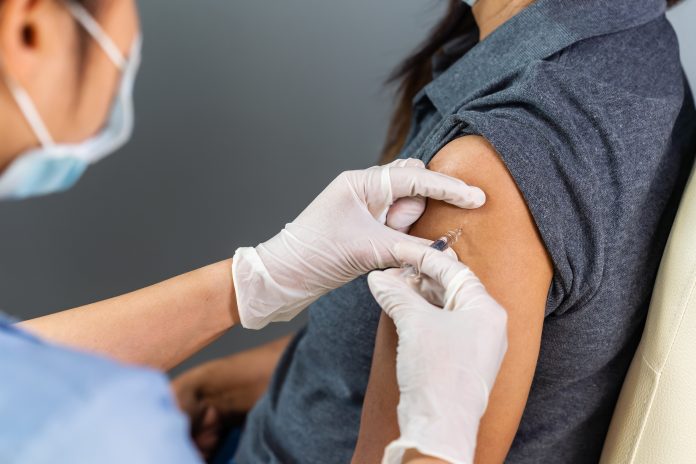
Recent data published by the UK’s independent medicines regulator assures the Pfizer/BioNTech and the Oxford University/AstraZeneca vaccines meet strict regulatory standards for safety
The latest data from the Medicines and Healthcare products Regulatory Agency (MHRA) shows that the Pfizer/BioNTech and the Oxford University/AstraZeneca vaccines continue to meet strict regulatory standards for safety.
Over 10 million doses of the Pfizer/BioNTech and the Oxford University/AstraZeneca vaccines have been administered across the UK so far and the vast majority of reported side effects are mild and short-lasting.
Side effects
The benefits of the COVID-19 vaccines continue to outweigh the risks of any known side-effects, such as fatigue, sore arms and mild ‘flu-like’ symptoms – a normal immune response to vaccines.
The data presents 22,820 reports of suspected side effects – an overall reporting rate of 3 in 1,000 doses.
Dr June Raine, MHRA Chief Executive, said:
“Vaccines are the most effective way to protect against COVID-19 and save lives and prevent serious complications from this terrible virus.
“The data we have collected provides further reassurance that the COVID-19 vaccines are safe and continue to meet the rigorous regulatory standards required for all vaccines. We remain confident that the benefits of these vaccines outweigh any risks.
“Our priority is to ensure the public have safe and effective vaccines and we will continue to analyse, monitor and review all the safety data for these vaccines.
“I’d like to thank everyone who has reported a potential side effect to us – every report matters.”