Targeted stem cell research at the University of California, Irvine could one day eradicate glioblastoma brain tumours, explains John Lowengrub
Glioblastoma (World Health Organization Grade IV Astrocytoma, GBM) is the most aggressive and dangerous brain tumour. More than 10 thousand GBM patients die each year in the United States, while the median patient survival for GBM is less than 12 months (1). It remains a challenge to eradicate glioblastoma due to its high heterogeneity, intense vascularization and innate treatment resistance.
Over the past decades, various therapies have been studied and tested clinically. The standard of care treatment for newly-diagnosed GBM patients consists of surgical resection of the tumour, followed by combined radiation and chemotherapy (2). Unfortunately, almost all the patients progress during or shortly after the treatment, and tumour recurrence is frequently observed. The FDA-approved antiangiogenic therapy for recurrent glioblastoma patients also fails to improve the survival rate (3). This prompts a thorough understanding of the heterogeneity in glioblastoma, and the development of new, personalised and more effective cancer therapies.
In glioblastoma, the glioma stem cell (GSC) hierarchy has been found to play a crucial role in tumour development (4). Tumours with high GSC population are more aggressive, and GSCs are believed to be responsible for tumour resistance to radiotherapy and chemotherapy (5). Primary human endothelial cells (ECs) are found to help to maintain the GSC pool and increase the size of tumour spheres, suggesting positive feedback from ECs to stem cells (6). Furthermore, GSCs have been found to transdifferentiate into bona-fide vascular endothelial cells (GEC), which inherit mutations present in GSC, support GSC proliferation and self-renewal, and are resistant to traditional anti-angiogenic therapies (7).
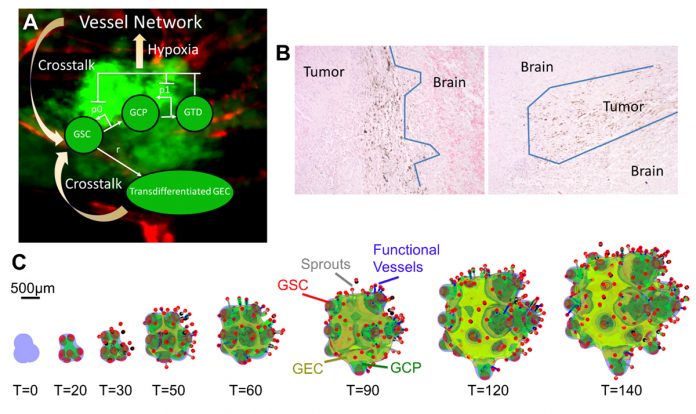
In recent work, Yan et al. (8) have developed a 3D hybrid continuum-discrete model of GBM that accounts for GBM cancer stem cells and their progeny, feedback among the different cell types, and tumour-induced neovascularisation (Fig. 1A). Their model, which builds upon earlier work by Cristini and Lowengrub (9), shows that GSCs self-organise and GSC clusters emerge at tumour boundary (Fig. 1C). These GSC clusters generate invasive fingers with GSCs staying at fingertips and differentiated cells trailing behind, which has been observed clinically (Fig. 1B). Vessel sprouts form near the tumour boundary, then grow and anastomose into functional vessels that are connected to host vasculature and supply nutrients to a tumour. GECs form a network within the hypoxic core, consistent with experimental findings. In the tumour interior, the vessel density and the level of EC-GSC crosstalk is highest, resulting in multiple new GSC clusters. Consequently, cell proliferation is enhanced and the tumour volume grows rapidly. Since the EC-GSC crosstalk has fuelled the tumour growth, it should be targeted in anti-tumour therapies.
Growth reduction
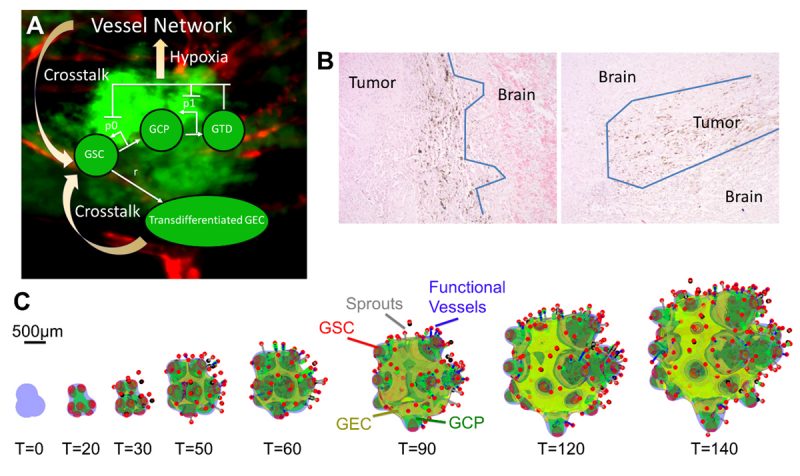
Yan et al. first targeted the crosstalk using an extreme scenario in which the vasculature is removed completely from a tumour and the microenvironment, and new vessels are not allowed to form. When this anti-angiogenic therapy (AA) is applied continuously, the overall volume growth is reduced. The growth is driven by the GSC clusters at the fingertips, which are less affected by the removal of the vessels. Consequently, the fingers continue elongating and penetrating the host. Thus, AA considerably increases tumour invasiveness (Fig. 2, top), consistent with experimental and clinical findings (10).
Since GSC clusters near the tumour boundary play an important role in tumour invasion driving invasive fingering, Yan et al. target these GSCs by modelling an anti-GSC therapy where the background vasculature continuously releases GSC differentiation promoters. Combining this differentiation therapy (Diff) with anti-angiogenic therapy, which has been shown to slow down tumour growth, also reduces invasiveness (Fig. 2, AA+Diff). However, the tumour cannot be eradicated since GECs are distributed at the tumour boundary and protect GSC from differentiation (Fig. 2, yellow). When a tumour is treated additionally by an anti-mitotic therapy (e.g. chemo- and radiotherapy), the GECs still cover the tumour boundary and support GSCs (Fig. 2, AA+Diff+AM). Thus, Yan et al. turn to a combinatorial therapy that incorporates anti-GEC, anti-GSC (differentiation), anti-angiogenic, and anti-mitotic (e.g. chemotherapy) treatments. This combinatorial therapy is shown to be able to eradicate a tumour (Fig. 2, bottom), without recurrence even after the treatment is stopped, allowing the patient to enjoy true, long-lasting remission.
In these modelling studies, Yan et al. have shown that targeting the EC-GSC crosstalk may hold promise as a novel anticancer therapy. In addition, using combinatorial therapies to target transdifferentiated vascular endothelial cells present in glioblastomas could eradicate these deadly brain tumours without recurrence. One candidate agent is potentially an EGFR blocker. EGFR is involved in proliferation, differentiation, migration and angiogenesis regulation in many glioma tumours (11) and the EGFR mutation is also present in the GECs (1)2. Therefore, blocking EGFR could effectively target GECs, among other targets. The latest generation of EGFR inhibitors, such as irreversible tyrosine kinase inhibitors (dacomitinib) effectively reduces the tumour volume in animal models by decreasing the GSC population by enhancing differentiation (13). Therefore, Yan et al. suggest that a brain-penetrant EGFR inhibitor potentially matches the need for a differentiation promoter, anti-GSC and anti-GEC agent, which could be combined in future clinical trials with both anti-angiogenic therapy and chemotherapy.
Contributors:
Huaming Yan1,7, Mónica Romero-López2,
Lesly I. Benitez4, Kaijun Di4,9, Hermann B. Frieboes5,6, Christopher C. W. Hughes2,4,7,8, Daniela A. Bota8,9,10, Vittorio Cristini11, John Lowengrub1,2,7,8
1 Dept. of Mathematics, University of California, Irvine, CA 92697
2 Dept. of Biomedical Engineering, University of California, Irvine, CA 92697. Current address: Orthopedic Surgery, Stanford University, Redwood City, CA 94063
3 Dept. of Developmental and Cell Biology, University of California, Irvine, CA 92697
4 Department of Molecular Biology and Biochemistry, University of California, Irvine, CA 92697
5 Department of Bioengineering, University of Louisville, Louisville, KY 40292
6 James Graham Brown Cancer Center, University of Louisville, Louisville, KY 40292
7 Center for Complex Biological Systems, University of California, Irvine, CA 92697
8 Chao Comprehensive Cancer Center, University of California, Irvine, CA 92697
9 Department of Neurology, University of California, Irvine, CA 92697
10 Department of Neurological Surgery, University of California, Irvine, CA 92697
11 Center for Precision Biomedicine, Brown Foundation Institute of Molecular Medicine, University of Texas Health Science Center at Houston (UTHealth) McGovern Medical School, Houston, TX 77030, USA
Footnotes
- Ostrom, Q.T., et al., CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol, 2015. 17 Suppl 4: p. iv1-iv62.
- Stupp, R., et al., Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med, 2005. 352(10): p. 987-96.
- Field, K.M., et al., Bevacizumab and glioblastoma: scientific review, newly reported updates, and ongoing controversies.
Cancer, 2015. 121(7): p. 997-1007.
- Lathia, J.D., et al., Cancer stem cells in glioblastoma. Genes Dev, 2015. 29(12): p. 1203-17.
- Bao, S., et al., Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res, 2006. 66(16): p. 7843-8.
- Calabrese, C., et al., A perivascular niche for brain tumor stem cells. Cancer Cell, 2007. 11(1): p. 69-82.
- Ricci-Vitiani, L., et al., Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature, 2010. 468(7325): p. 824-8.
- Yan, H., et al., 3D Mathematical Modeling of Glioblastoma
Suggests That Transdifferentiated Vascular Endothelial Cells
Mediate Resistance to Current Standard-of-Care Therapy. Cancer Res, 2017. 77(15): p. 4171-4184.
- Cristini, V. and J.S. Lowengrub, Multiscale modeling of cancer: An integrated experimental and mathematical modeling
approach. 2010, Cambridge, UK: Cambridge University Press.
- Paez-Ribes, M., et al., Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell, 2009. 15(3): p. 220-31.
- Roth, P. and M. Weller, Challenges to targeting epidermal growth factor receptor in glioblastoma: escape mechanisms and combinatorial treatment strategies. Neuro Oncol, 2014. 16 Suppl 8: p. viii14-9.
- Emlet, D.R., et al., Targeting a glioblastoma cancer stem-cell population defined by EGF receptor variant III. Cancer Res, 2014. 74(4): p. 1238-49.
- Zahonero, C., et al., Preclinical Test of Dacomitinib, an
Irreversible EGFR Inhibitor, Confirms Its Effectiveness for Glioblastoma. Mol Cancer Ther, 2015. 14(7): p. 1548-58.
- Kaijun Di, M.E.L., Daniela A. Bota, TRIM11 is over-expressed in high-grade gliomas and promotes proliferation, invasion,
migration and glial tumour growth. Oncogene, 2013. 32(42): p. 5038-47.
Please note: this is a commercial profile
John Lowengrub
Chancellor’s Professor of Mathematics, Biomedical Engineering, Chemical Engineering & Materials Science
Director, Interdisciplinary Graduate Program in Mathematical,
Computational and Systems Biology
540H Rowland Hall
University of California, Irvine
Mobile: 949-751-9700
Tel: 949-824-8456
Fax: 949-824-7993