Secarna Pharmaceuticals, the next-generation antisense drug discovery and development company, is developing novel approaches to successfully target Neuropilin 1 (NRP1), a promising target for the treatment of cancer
The idea of using the immune system to fight tumors can be traced back to 1891, with Dr William Coley, a pioneer in immunotherapy. Today, our understanding of the immune system has skyrocketed, and various cancer immunotherapies, such as immune checkpoint inhibitors (ICIs), cancer vaccines, and CAR T-cell therapies, have been developed that harness the immune system to recognize and destroy tumor cells effectively.
Despite these successes, most patients fail to achieve an objective clinical response. A key reason for this is the ability of tumors to develop resistance mechanisms. Consequently, significant efforts in the field have centered on evaluating combination strategies and searching for novel approaches.
Neuropilin 1: A promising target in oncology
Recent studies have indicated that Neuropilin-1 (NRP1), a protein located on the surface of cells, is involved in various tumor-promoting functions. The protein is expressed on many different types of cells within the tumor microenvironment. High NRP1 expression is correlated with a poor prognosis in several types of cancer and negatively associated with a response to anti-PD1 ICI therapy.
NRP1 is a co-receptor that is involved in a variety of cell signaling pathways. These pathways regulate, for example, the formation of new blood vessels which is important for the supply of the tumor with nutrients. Furthermore, they regulate cellular migration, a prerequisite of tumor metastasis. Migration of immune cells into the tumor often results in their reprogramming into immunosuppressive and tumor-supporting cells. Examples of immunosuppressive immune cells that NRP1 regulates are M2-polarized macrophages and regulatory T cells (Tregs). These diverse functions are mediated by separate parts (domains) of the protein, making it very difficult to develop drugs that can target all domains simultaneously and hence block all of NRP1’s pro-tumorigenic processes at the same time. (1)
Secarna’s SECN-15: The first antisense oligonucleotide to target Neuropilin 1
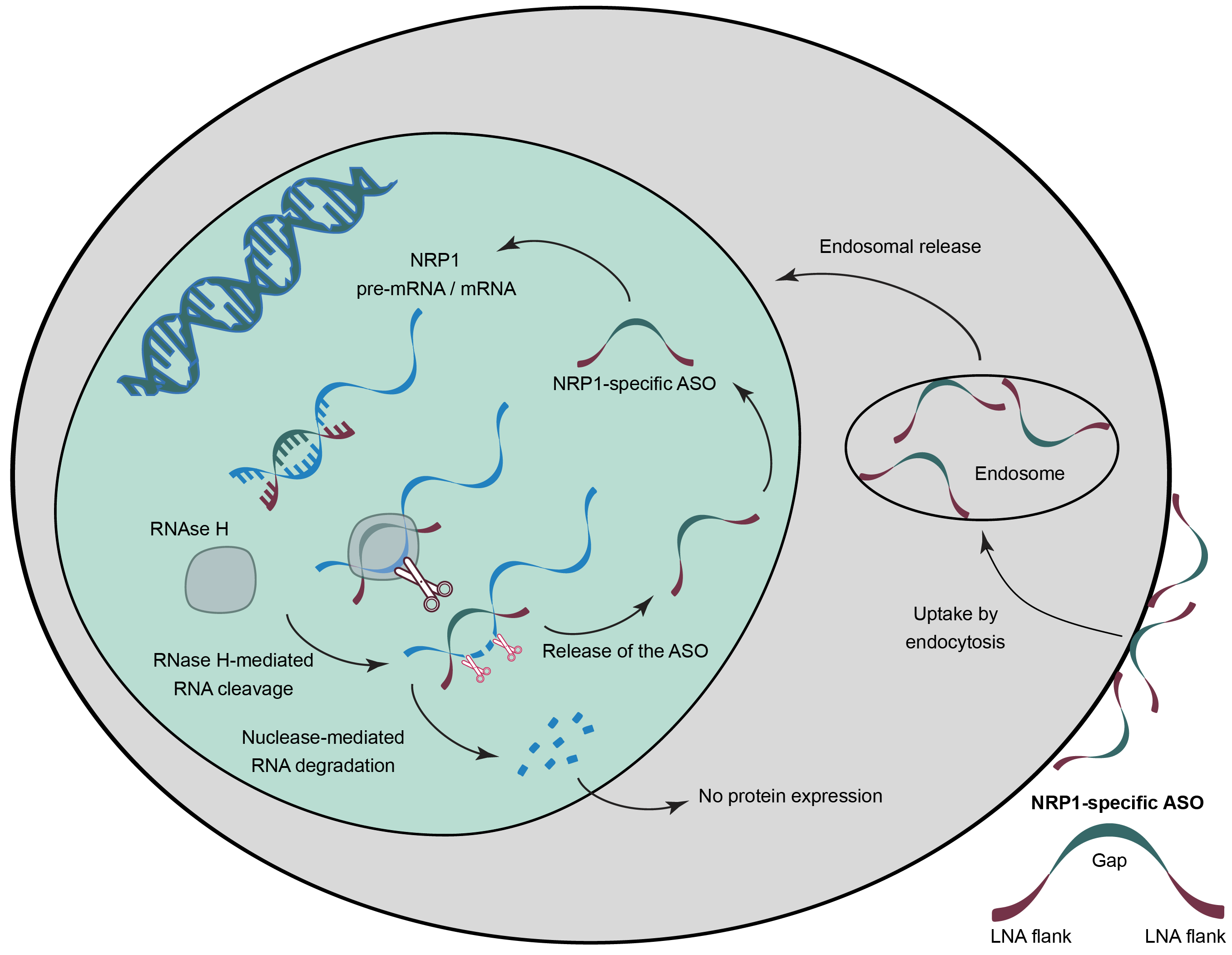
With its next-generation proprietary antisense oligonucleotide (ASO) platform (LNAplus) and its OlygofyerTM bioinformatics system, Secarna has developed SECN-15, a highly potent ASO to specifically target Neuropilin 1.
ASOs, in contrast to other drug modalities, have the potential to block all functions of NRP1 simultaneously by targeting at the pre-mRNA level, that is, a step prior to the formation of the protein. The Company has demonstrated that it can selectively and with high potency target NRP1, showing a higher than 90% knockdown of the protein expression in vitro.
SECN-15 shows impressive potential: As single therapy and in combination with anti-PD-L1 antibody
To further explore the effect of SECN-15 in animal tumor models, Secarna analyzed NRP1 protein levels in different cell types within the tumor, including immune cells with immunosuppressive functions, and could show that levels were significantly reduced in animals treated with SECN-15. With this, Secarna demonstrated that its ASO is effective in vivo and can target the relevant cell types for effective treatment.
This translated into a potent anti-tumor activity in animals. A striking suppression of tumor growth rates was achieved, together with an increase in survival. In many cases, complete and durable tumor eradication was seen when treatment with SECN-15 was combined with an anti-PD-L1 antibody. Animals that were tumor-free after treatment were exposed a second time to the cancerous cells but did not develop a tumor, indicating the establishment of anti-tumor immunity in treated mice.
Transforming the treatment of cancer
SECN-15 has shown that simultaneous inhibition of each of NRP1’s pro- tumorigenic functions via knockdown of its expression is a favorable treatment modality, potentially overcoming the limitations of alternative approaches. Currently, two other compounds (small molecules and antibodies) in pre-clinical stage are being developed to target NRP1 and/or its associated pathway. None of these programs address all functions at once. Hence, LNA-modified ASOs targeting Neuropilin 1 expression have the potential to become a new treatment option in tumors that do not respond to current treatments.
Further mechanistic research will be instrumental in translating these findings for the clinic.