With a changing climate, animal, insect, and plant species will either adapt or die under new environmental and climate conditions – this brings new challenges in the study of vector-borne infectious diseases
Species, including humans, around the world have no other option than to respond to a changing climate. The species at highest risk of going extinct may be less able to adapt due to slower reproduction strategies or an inability to expand their range and migrate. Other species readily adapt to environmental change, however; these include mosquitoes, ticks and other vectors for infectious diseases. Now is the time to research how species will change, so we can better predict the when and the where. Researchers at Los Alamos National Laboratory in the Southwest U.S. are now investigating how landscape and climate change will impact vector borne diseases in the future for the Americas.
Vector-borne diseases such as dengue and malaria have complex epidemiology and ecology that are closely tied to both the environment and human behaviours. The disease “system” changes with a fluctuating environment and is a series of interactions between hosts, reservoirs, vectors and pathogens. This creates significant challenges for scientists who try to predict how vector-borne diseases will spread in the future. Developing a forecast of how the spread of vector-borne diseases will respond to climate change requires experts from different backgrounds to work together. To improve forecasting of disease outbreaks in response to climate change, the Los Alamos National Laboratory team is made up of computer and data scientists, disease ecologists, mathematicians, earth systems modellers, and epidemiologists studying outbreaks of high-profile diseases. These include influenza, Zika, dengue, and the current COVID-19 pandemic (Bartlow et al. 2019).
In North America, the number of disease cases resulting from infected mosquitoes, ticks, and fleas more than tripled between 2004 and 2016 (Centers for Disease Control and Prevention 2019). Mosquitos are probably the best known and most well-studied disease vector globally. In addition to carrying the Plasmodium parasite that causes malaria, mosquitoes also carry and transmit other serious illnesses such as yellow fever, Japanese encephalitis, West Nile virus, and dengue fever.
Vector-borne diseases account for more than 17% of the total cases of infectious disease worldwide, and cause over 700,000 deaths every year (Roser and Ritchie 2019). In 2019, more than 85 countries reported malaria, and an estimated total of 229 million cases occurred globally. The mosquito genus Anopheles is the primary vector for malaria, while the genus Aedes and Culex are primarily responsible for transmitting the viruses that cause dengue fever, West Nile virus, chikungunya, Zika virus and others.
Data challenges and solutions
Understanding how vector-borne infectious diseases are changing with climate comes with many data challenges. First, it requires working with large data sets at different scales and across many disciplines to capture human, virus, vector and ecological dynamics. Second, it requires an understanding of how to effectively and accurately combine different types of information and models with a variety of spatial and distributions. Lastly, understanding the biology of mosquitoes and how they are responding to subtle habitat changes means working with mosquito researchers on the ground who are collecting data on mosquito behaviour and populations. Forecasting mosquito populations and infectious diseases requires seamless collaboration between researchers in the field and behind the computer/in the lab.
Despite these challenges, at the right scale, robust forecasts can be created and used in the future. Different streams of data must be collected, including: The number of cases of a disease in humans or sentinel species like birds, habitat types and locations around the area, mosquito species and populations, and of course weather and climate conditions. Our multidisciplinary, Climate Integrated Model of Mosquito-borne Infectious Diseases (CIMMID) team at Los Alamos National Laboratory, is working to create a system capable of better forecasting by coupling spatial models with earth system models.
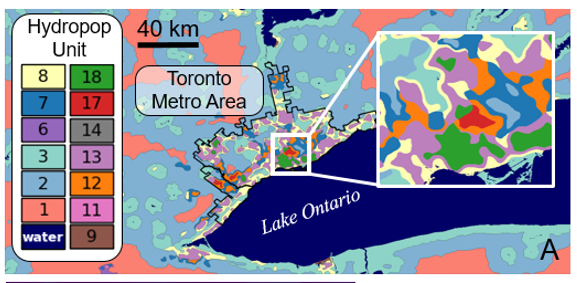
The spatial models factor in how hydrology, mosquito abundance, and human populations intertwine and are called a hydropop units. This modelling system could also be used to predict vector-borne disease outbreaks after environmental catastrophes such as hurricanes, floods, and earthquakes. Mosquito borne diseases and other infectious disease outbreaks are common after such natural disasters, leading to even more stress, illness and death. Knowing what diseases might be on the horizon can help responders and public health officials better prepare for these emergency situations.
Vectors and the diseases they carry will adapt to future conditions
The risk of a particular disease and potential future outbreaks can be mapped and forecasted through epidemiological modelling. Knowing where limited resources are most needed for future detection and mitigation of disease can better inform bio-surveillance networks. These networks can include mosquito surveillance that is currently being carried out in most regions at some level. Both environmental and climatic conditions have been shown to accurately predict future risk of vector-borne diseases. Different types of data can be used to identify signatures for mosquito borne disease outbreaks in both space and time (Castro et al 2021).
Because vectors respond much more quickly to factors of environmental change such as rainfall and temperature, these factors can be used to predict risk of vector-borne diseases months in advance. Changes in climate, habitat, hydrology, and population for both humans and animals are all considered when modelling disease risk. However, the sheer number of systems and factors involved creates a level of complexity that can be difficult to make sense of without the right data at the right scale.
Beyond mosquitoes, humans, agricultural animals, wildlife, and plants also respond to rapidly changing environmental conditions. Other important factors to consider in the propagation of vector-borne infectious diseases include: The movement of people and their animals, the availability of effective and prophylactic drugs, elements of human infrastructure such as air conditioning or dustbin collection, access to public health services; and, lastly, political conflicts and stability in a region.
References
Bartlow W.A., Manore C., Xu C., Kaufeld A.K., Del Valle S., Ziemann A., Fairchild G., and J. Fair. 2019. Forecasting zoonotic infectious disease response to climate change: mosquito vectors and a changing environment. Veterinary Sciences 6. doi: 10.3390/vetsci6020040
Castro, L.A., Generous, N., Luo, W., Pastore y Piontti, A., Martinez, K., Gomes, M.F.C., Osthus, D., Fairchild, G., Ziemann, A., Vespignani, A., Santillana, M., Manore, C.A., Del Valle, S.Y., 2021. Using heterogeneous data to identify signatures of dengue outbreaks at fine spatio-temporal scales across Brazil. PLOS Neglected Tropical Diseases 15, e0009392
Centers for Disease Control and Prevention. Illnesses on the rise from mosquito, tick, and flea bites. 2019 p. https://www.cdc.gov/vitalsigns/vector-borne/index
Roser, M. and H. Ritchie. 2019. “Malaria”. Published online at OurWorldInData.org. Retrieved from: https://ourworldindata.org/Malaria
Please note: This is a commercial profile